COMPANY
INSIGHT

EXERA® precision medical wire and wire-based solutions
Sandvik develop, manufactures and refine precision medical wire and components under the brand name EXERA®. Our in-house die craftsmen and women utilize a combined experience of over 100 years to put the precise art of die making at your service. It’s this type of painstaking attention to detail that makes us unique.
We seek to provide value at every step of the medical component design and manufacturing process. From precision tolerance coating to multi-filar micro cables, we strive to provide you with not only a supplier, but a business partner.
Naturally you want the best possible materials and at Sandvik quality is paramount. We leverage time-tested procedures to maintain the highest standards for our EXERA® fine medical wire products and components. We also understand the importance of agility in our design process as we continually implement lean initiatives to increase quality and decrease variability. We are ISO13485, 9001, 14001 and OHSAS 18001 certified.
Read more about EXERA® fine medical wire and wire-based components https://exera.sandvik
Let us inspire you and create the products of the future
As your partner in the design process, we strive to integrate operations and maintain responsive and comprehensive interaction with you. Together, we can design a process and product that cannot be found anywhere else in the world. Let us inspire your product innovation for the future.

Katina has long experience in the company and has been part of developing products for various sensing solutions.
Materials and components we offer:
Materials we offer: Stainless steel grades, MP35N, copper based alloys, additional medical and precious metal grade alloys such as Pt, PtIr and Au and multiple resistance alloys.
Product forms including wire, fine wire, coated wire, flat wire, plated wire, coils, guidewire material, multi-filar wire forms as micro cables or stranded constructions.
We produce ultra-fine wire, round wire sizes range from 0.010 to 10 mm (0.00039 to 0.394 in.), for most alloys. Other sizes available upon request.
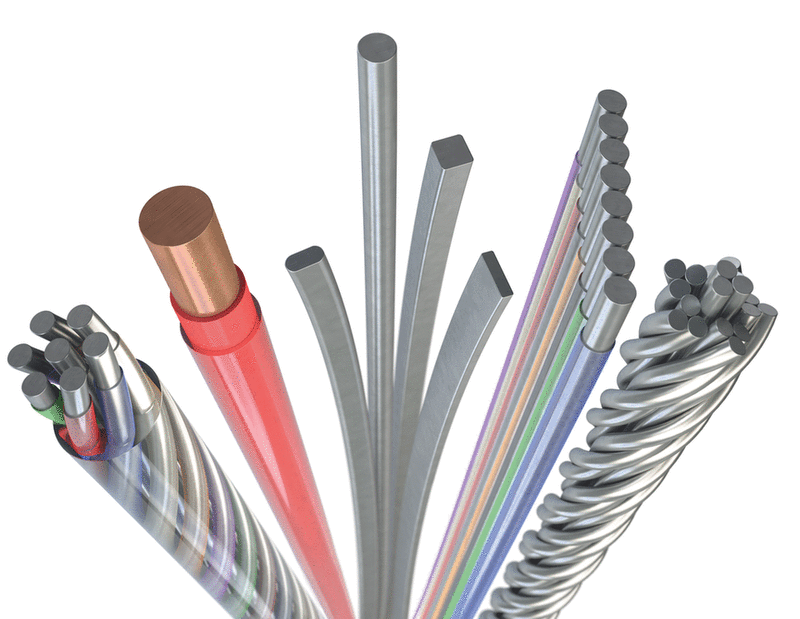
We can make various product forms, sizes and combinations. Tell us what you want and we can find a solution together.
Ribbon wire thickness ranges from 0.013 to 0.75 mm (0.0005 to 0.03 in) with aspect ratios up to 10:1. Larger aspect ratios up to 40:1 may also be available upon request.
The alloy or base material can be supplied with or without a polymer coating in various colors. Typical thickness is 2,5 µm – 25 µm (.0001” - .001”), thicker on request. Surface finishes can be modified with gold, nicker or silver plating or provided with an oxidized layer (when not a noble metal).

Another key competence at the Sandvik Palm Coast facility is coating where we besides traditional polymer and PTFE coatings also can offer custom formulations.
We also support our customers to produce wire-based components. From precision tolerance coating to multi-filar micro cables, we can provide you the best possible solution for your specific medical device application. We can help you conceive and implement innovative materials solutions that can make the manufacture of your device highly reliable and meet the strict quality requirements.
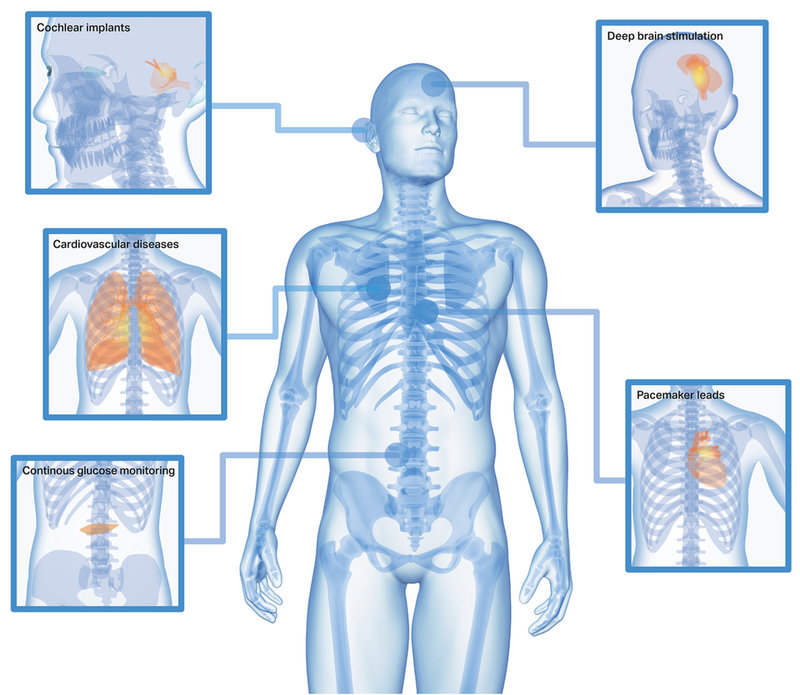
The wire-based applications are used for applications cardio/cardio vascular, neuro/spine, glucose monitoring and cochlear implants. Read more here
About Sandvik Group
Sandvik is a world-leading developer and manufacturer of advanced stainless steels and special alloys and a fully integrated producer of metallic precision materials for medical applications such as gastrointestinal, orthopedic, spinal, neurophysiological, electrophysiological, vascular, and cardiovascular. We develop, manufactures and refine precision medical wire and components under the brand name EXERA®. Read more about our medical wire and wire-based components
The EXERA® fine medical wire and components are produced at the Sandvik Palm Coast production unit in Florida, USA. Also, EXERA® fine medical wire and components are sold globally utilizing the footprint and logistics of Sandvik Group. With us you get the advantage of working with a small, agile, custom, precision wire manufacturer in the Palm Coast production unit, and the backing of the globally integrated and resource rich Sandvik Group.
Sandvik is committed to using engineering and innovation to make the shift that will drive more sustainable business. Our aim is to lead this shift in our industry and be the innovative business partner for our customers by making sustainability part of every aspect of business, delivering value for everyone. Read more about our sustainability work at Sandvik
We invest 3,5 billion SEK in R&D each year and we have about 7300 active patents. Sandvik is a Swedish company founded 1862 in Sandviken giving us over 150 years of experience to share with our customers. Today the headquarter is based in Stockholm, the capital of Sweden. We operate in over 150 countries worldwide with about 42,000 employees. We have a strong commitment to enhancing customer productivity, profitability and safety. Read more about Sandvik Group
Learn more about Sandvik in three minutes

