Biocompatible Coatings to
Enhance Your Medical Device
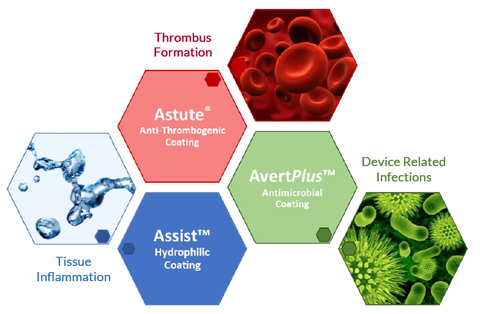
Advancements in medical devices have demanded innovative coatings to enhance the biological acceptance and reduce the challenges faced when artificial materials are in contact with the body.
BioInteractions have developed three unique polymer coatings to help with these challenges:
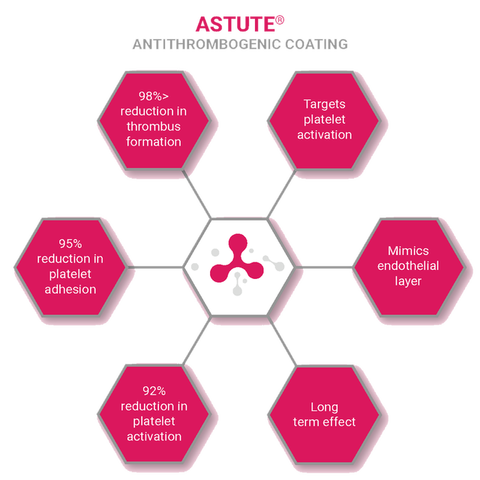
Astute®
Anti-thrombogenic Coating
Triple-Endothelial Action
Astute functionalises an active component within its polymer backbone and is combined with passive components to actively prevents a thrombus from forming.
Astute® Triple Endothelial Action:
- Actively negates platelet activation
- Prevents protein and cellular deposition
- Non-leaching coating to give long term efficacy
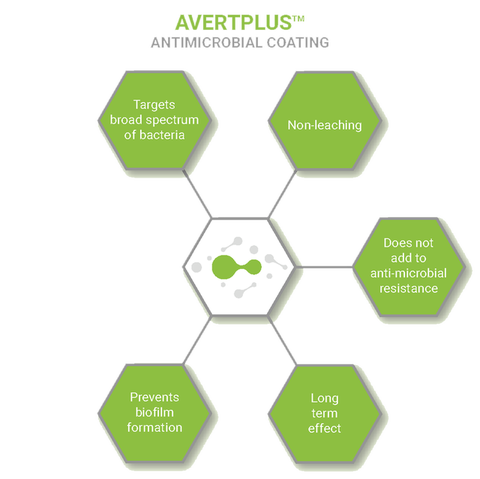
AvertPlus™
Dual Active Contact Kill Mechanism
Antimicrobial Coating
AvertPlus™ reduces device related infections and targets biofilm formation. The active contact kill mechanism is paired with a passive process to reduce the presence of bacteria on the surface
AvertPlus™ Dual Active Contact Kill Mechanism:
- Active disruption of a broad-spectrum bacteria that results in lysis
- Prevents deposition and adhesion of proteins
- Non-leaching coating gives long term efficacy throughout lifetime of the coating
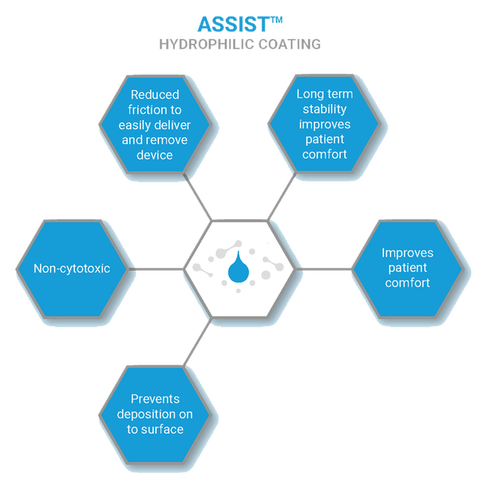
Assist™
Enhanced Lubricious Activity
Hydrophilic Coating
Assist™ minimises the obstacles faced at the device-body interface. The polymer coating provides a highly hydrophilic surface which reduces friction as well as preventing protein binding and platelet deposition to improve patient well-being.
Assist™ Enhanced Lubricity:
- Hydrophilic properties as well as prevents deposition onto the surface
- Non-leaching, non-cytotoxic and stable coating
- Available as UV Cured or Heat Cured
Commitment to Care Services
Our Commitment to Care Service (C2C) builds on our expertise in facing a broad range of challenges. We have combined our innovative coatings with optimised application processes and support services as a complete solution for our partners. We have discovered the impact of applying the coating on the result of the quality and efficacy of the coating. We are able to develop and optimise a coating process as well as provide the coating capabilities in our cleanroom facilities for our customers.
We have extended our C2C services to include testing methods to highlight the benefits of using our coating technology. This can be used to help support regulatory submissions. We have expertise in helping our partners through the submission process helping to reduce significant challenges during this process
Our in-depth understanding of the range of challenges faced by materials, provides us with an exclusive advantage to help our partners. Our coating technologies provide innovative advantages to the device and helps to Assist™ in improving patient well-being.
Back To Top