PI Ceramic develops and produces standard and customised piezoelectric actuators and sensor components for applications such as surgical power ultrasound, vaporizers and implantable subassemblies. The company supplies piezoceramic solutions for ultrasonic fluid and gas metering, pumps and valves as well as dispensing systems and miniaturized components for devices with limited assembly space, for example endoscopic applications.
Our PI UK subsidiary in Cranfield is hosting a Piezo Academy on 17th March 2019 on “Piezo Ceramic Applications in the Medical World”. Sign up here for a day of inspiring insights into piezo-driven MedTech applications.
Therapeutic Ultrasound with
Piezo Components
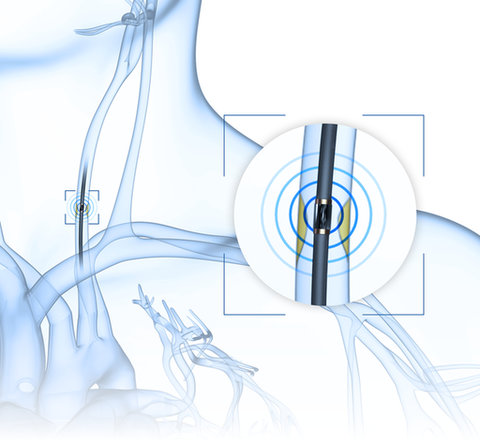
Ultrasound opens up new and innovative therapeutic possibilities that improve or even substitute many established procedures: Minimally invasive and gentle treatment methods with improved therapy success and fewer side effects can be realized using piezo components.
Therapeutic ultrasound refers to methods in which ultrasound is not only a means of diagnosis but also the core element of the therapy, e. g. tissue ablation, targeted drug delivery, ultrasound-assisted thrombolysis, and lithotripsy.
Find out more about piezo components in MedTech
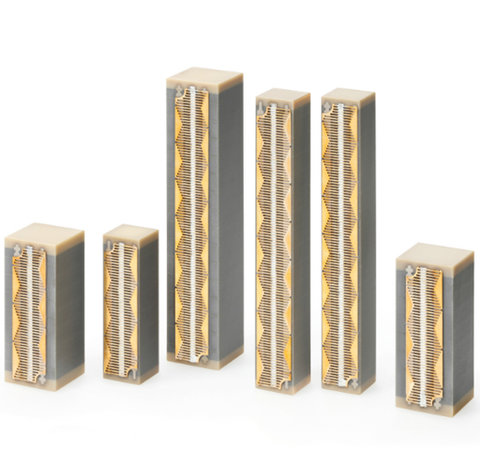
Pumping and dosing with PICMA® Multilayer Piezo Actuators
The patented piezoelectric PICMA® Multilayer Piezo Actuators are covered by an all-ceramic insulation layer and protected against humidity, resulting in a superior lifespan. Therefore, the monolithic PICMA® Multilayer Piezo Actuators achieve enhanced reliability even under extreme ambient conditions.
Due to their low operating voltage, instantaneous displacement and high forces, PICMAs can be used in various medical pumping and dispensing applications like precision dosing or cell sorting. Even in limited space, the miniaturised PICMA® Chip actuators enable extreme fast picoliter precise droplet generation.
Nevertheless, PICMA® Multilayer Piezo Actuators are available in various designs with different displacement modes. In addition to working with longitudinal displacement, various customised PICMA® Benders operate in two-dimensional motion and execute a millimetre displacement so they can be used for vale and pumping functions suitable for lab-on-a-chip devices.
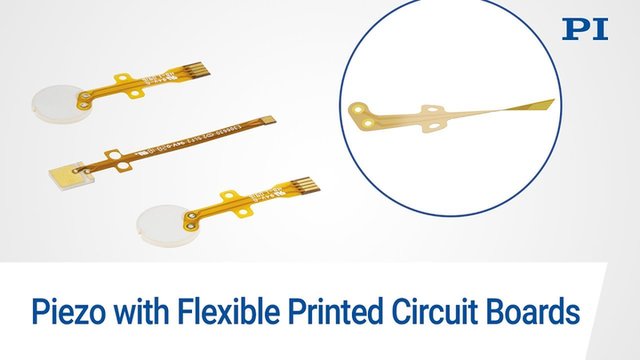
Plug and Play:
Piezo Components with Flexible PCBs
PI Ceramic not only develops application-specific piezo components but also designs printed circuit boards (PCBs) and connects the two elements by soldering or glue.
This saves customers a time-consuming and fault-prone step of production. If carried out without the required expertise, the soldering process can affect the piezoelectric properties of the components.
PI Ceramic offers this ready-to-use option, ensuring a consistent product quality.
Customised piezoelectric
sub-assemblies and transducers
Due to PI Ceramic’s experience of glueing and soldering technologies, we also develop customised sub-assemblies and complete ultrasonic transducers in cooperation with the customer.
Our technological capabilities include contacting with wires and laces, glueing of components on membranes or substrates, as well as the production of small to medium series transducers.
All process steps are united under one roof and
the cleanroom environment offers best conditions for medical technology component manufacturing.
The PI ceramic transducers work with frequencies up to several megahertz. Therefore, they can be used for flow and filling level measurement as well as air bubble detection.
About PI Ceramic GmbH
PI Ceramic is a subordinate of Physik Instrumente (PI) GmbH & Co. KG and situated in Lederhose, Germany. It currently employs more than 330 staff, including 80 engineers, in piezo research, development and manufacturing.
For small series production, higher refinement, new technologies and special shapes, the PI Ceramic Tech Centre was created so customers can quickly and easily qualify samples for their specific projects.
BACK TO TOP