Implants
Not very re-Essureing:
how a contraceptive implant can ruin lives
All healthcare providers in the US were ordered to return any unused Essure sterilisation devices to manufacturer Bayer by the end of December 2019, after years of severe side effects reported by women fitted with the coils. Chloe Kent takes a closer look at what this has meant for Bayer, and what’s next for the patients who still have Essure inside of them.
E
ssure was approved for sale by the US Food and Drug Administration (FDA) in November 2002. The permanent sterilisation device consists of metal coils and is designed to be inserted vaginally through the cervix and uterus into the fallopian tubes. Once in place, the coils – made of polyester fibres, nickel-titanium, stainless steel and solder – block the passage of eggs into the uterus, and scar tissue gradually forms around the coils, sealing them in place.
The device was designed to remain in place for a lifetime, but thousands of women have since experienced severe side effects following their procedures. Many have resorted to hysterectomies in a bid to remove Essure from their bodies when other options proved inviable.
There have also been 49 reports of death linked to the device. Overall, 17 of these relate to 15 adult deaths and 23 relate to 20 incidences of miscarriage. A further five detail five incidences of infant death after live birth, two relate to separate incidences of ectopic pregnancies, one specifies a death but does not specify if it occurred before or after birth and another references an unknown number of deaths posted on social media.
The FDA placed a black box warning on Essure in November 2016, before limiting its sale in in April 2018 to healthcare providers who would use a checklist to warn patients of the risks. In July 2018, Essure manufacturer Bayer announced the product would no longer be sold in the US by December 2018. All unused devices were to be returned to the company by the end of December 2019.
A multitude of errors
Between November 2002 and December 2018, 32,773 medical device reports about Essure were submitted to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, most of which described more than one complication. Many of these symptoms are thought to be caused by autoimmune reactions to the metals Essure is made of.
FDA’s Manufacturer and User Facility Device Experience (MAUDE) database from 2002 - 2018
Pain: 26,244
Heavier or irregular periods: 13,114
Headache: 8,398
Fatigue: 6,912
Weight fluctuations: 5,853
Depression/anxiety: 5,175
Hair loss: 4,880
Hypersensitivity/rash: 4,807
Alongside symptomatic complaints such as these, the most frequently reported device problems were:
Alongside symptomatic complaints such as these, the most frequently reported device problems were:
Patient-device incompatibility (5,206),
Migration of the device or device component (3,410),
Dislodged or dislocated device (1,632),
Breakage, material fragmentation or fracture of the device (1,533),
Device operating differently than expected (1,058),
Malposition of the device (336),
Device being difficult to remove (341)
Device being difficult to insert (335).
Frequently reported device problems
Benefits vs. risks
Throughout the controversy, Bayer has maintained that its decision to discontinue Essure was purely financial, citing “inaccurate and misleading” publicity behind its plummeting sales.
In a statement issued to Medical Technology, the company says: “There are no changes in the safety profile or effectiveness of the product, and this is not a product recall. Women who currently have Essure in place may continue to rely on the device, and Bayer will continue to support Essure patients and providers.”
Bayer currently faces more than 17,000 lawsuits relating to the device, which say it failed to report serious side effects such as perforated organs and broken devices to the FDA.
One patient suing Bayer over her implant is Sarah Dzikowicz, who was fitted with Essure in September 2006. She went on to experience several issues which have been associated with the implant, including heavy and painful periods, unexplained weight gain, autoimmune issues such as chronic fatigue syndrome, dental problems, gastrointestinal symptoms and brain fog. Dzikowicz eventually underwent a vaginal hysterectomy in 2015 to remove the implant but found out in late 2019 that despite undergoing the procedure an Essure coil had somehow remained inside her body.
“There are about 80,000-100,000 people in the world that have brain stimulators.”
Dzikowicz says: “I started making connections after watching The Bleeding Edge a couple of years ago, and then reading through Facebook posts in the Essure Problems group. It was after talking with my attorney and explaining my history to her that she recommended I have an X-ray to see if I still have a coil in me.”
Despite being informed at the time of her hysterectomy that everything but her ovaries had been removed, Dzikowicz’s medical history made no direct mention of whether her fallopian tubes were still inside her body or not. As it transpired, both were not taken out during the hysterectomy. A January 2020 surgery finally saw them removed, the Essure coil going with them, five years behind schedule.
“So far I’m feeling better,” Dzikowicz says. “As of now, I don’t have the fatigue that I had before. We’ll see what happens from here.”
Bayer maintains that the benefit-risk profile of Essure has not changed in light of cases like Dzikowicz’s and continues to stand by the product’s safety and efficacy.
Drugwatch senior writer Michelle Llamas says: “I can't speak to Bayer's honesty, but it has always stood by the safety of its device and they maintain discontinuing Essure was purely a business decision, even when thousands of women reported complications. These women claim Bayer will never acknowledge the safety problems with Essure.”
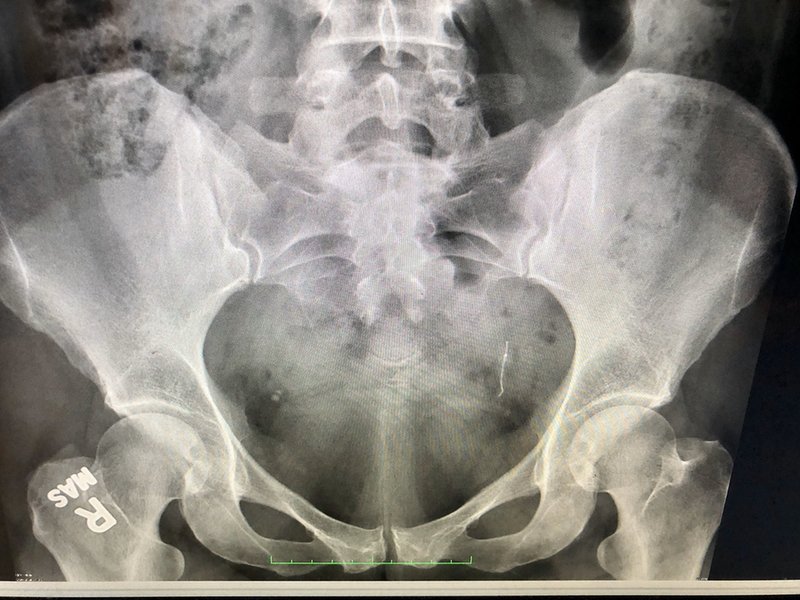
Sarah Dzikowicz was left with the device despite undergoing a hysterectomy to remove it. Image courtesy of Sarah Dzikowicz.
What Bayer did wrong, and where patients go next
Lawsuits claim that defects of Essure’s design can result in serious or permanent injury, and allege several account of negligence. These including: failing to warn about the risk of side effects, negligent training and risk management, and express breach of warranty.
Bayer is alleged to have used simulated training in how to fit the device in lieu of hands-on training, and did not verify that doctors had completed training before allowing them to implant the devices. It has also been accused of encouraging unqualified doctors to purchase two Essure sterilisation kits a month and ‘sell’ the procedure to their patients.
Bayer is also accused of failing to notify of 16,047 medical device complaints relating to the device and making false statements about Essure’s effectiveness and safety in its marketing materials.
Llamas says: “There are thousands of women who have filed lawsuits against Bayer, and those continue. These women also continue spreading awareness about the device and many still suffer from complications. The FDA is also continuing to monitor adverse event reports and extended Bayer's study period from three years to five.”
“The FDA does not recommend that women remove the device if they are not having problem.”
Essure was originally approved based on a premarket study, in which 745 women were enrolled. Only 92% of the participants were followed up for safety outcomes after a year, and only 25% after two years, despite the device being designed to be permanent.
The FDA ordered Bayer to conduct a post-market study starting in 2016, with the aim of enrolling 1,400 patients fitted with Essure coils and 1,400 patients who underwent laparoscopic tubal litigation, to compare and contrast their conditions after the procedure. However, due to the company’s market exit the targets were scaled back significantly to total 1,200 , with 329 patients currently recruited in the Essure wing of the study and 673 in the tubal litigation wing.
The device has been discontinued but not recalled, meaning no action is required from women fitted with Essure who aren’t experiencing any ill effects. The FDA does not recommend that women remove the device if they are not having problems, and advises anybody experiencing Essure problems report any adverse events on its site.
In the meantime, patients like Dzikowicz wait in limbo, to see what the courts will rule.