RPMC Lasers has partnered with LDX Optronics since the late ’90s. Over the course of our nearly 25-year relationship with LDX, we have seen their multimode diode laser capabilities grow to encompass sources ranging from 400-nm to 1900-nm and power levels up to 40-W. On top of all of that, the most impressive thing about LDX is its packaging expertise, which allows them to offer an incredibly diverse range of standard laser diode packages as well as customized assemblies.
Click here for more infomation
- High Power Multimode Laser Diodes
- Output Power - 100 mW to over 40 watts
- Wavelength - 400nm to 1900nm
- Standard and custom Packages
- Open Heatsinks - C-mount, Chip on Submount, Q-mount
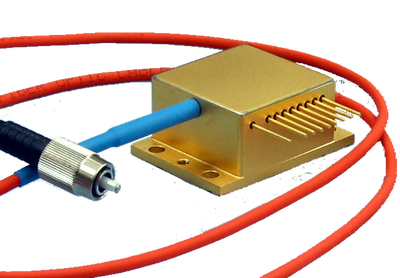
- Sealed Packages - 9mm, TO-3, HHL
- Fiber Coupled Packages – 2- pin, 9mm, HHL
- Options - Micro-Lensing, Aiming Beam, Photodiodes
We strive to provide lasers of the highest quality. Every laser wafer undergoes extensive qualification and life testing prior to acceptance. Each device is thoroughly burned-in and qualified prior to shipment.
It is our desire to provide customers with performance data so that they can make informed decisions. LDX is pleased to supply performance and lifetime lifetest data on any/all of the devices produced. The lifetime of the devices can range from >20,000 hours for the more common 808, 940, and 980 nm devices to shorter lifetimes for some of the more esoteric wavelength diode lasers. We also offer laser package drawings and typical laser performance data. Please visit RPMC Lasers for more information.

RPMC Lasers Inc
8495 Veterans Memorial Pkwy
O’Fallon MO 63366
(636) 272-7227
For more information on any of the over 200 standard laser diode configurations currently listed on our website you can, click here.



