As the world’s premier manufacturer of photochemically etched metal components, Micrometal has consistently pushed the boundaries of what is possible with chemical etching.
Our unique variation of the photochemical etching process, which includes use of an ultra-thin liquid photoresist and ultra-high resolution photomask tooling, enables us to produce customized parts that would otherwise be impossible to manufacture.

Our continuous reel-to-reel etching line allows Micrometal to supply nearly endless strips of components that are ideally configured for integration into high-volume manufacturing lines, with each component being completely identical to one another. If necessary, Micrometal can also provide individually packaged components that are ready for use.
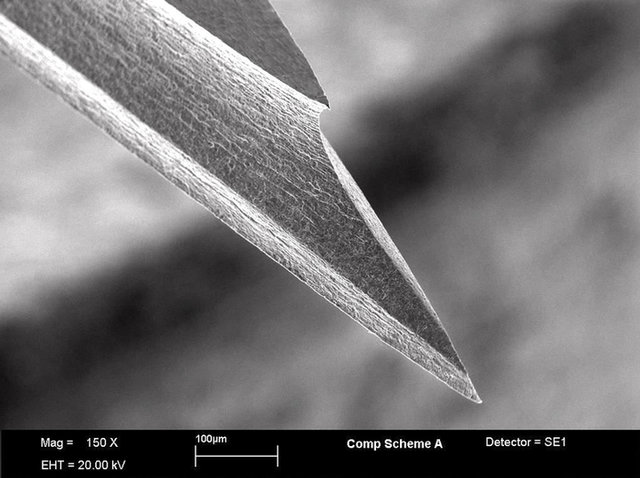
Many medical devices and applications require small metal components that demand very specific characteristics.
These can include:
- Ultra-High precision
- Very thin material
- Unique physical features
- Unique surface textures
- Identification features
- Extreme consistency part-to-part
- Ease of integration into the final device
Imagine specialty surgical blades that are pre-sharpened, directly from the etching process, or lancets with specific features etched in 3-D within the thickness of the material itself. How about metal filters with conical-shaped holes?
Our process provides levels of precision and design freedom that are impossible with other methods such as laser cutting, stamping, or EDM, while offering high-volume production capacity and cost efficiency.

Micrometal has years of experience in providing the most challenging metal components for medical devices and surgical applications. We and our subsidiaries can work with a variety of materials including stainless steel, aluminum, copper, titanium, etc.
Our design team works with you to fully understand the function and intent of YOUR application, and design a customized solution that is the ideal combination of performance, cost, quality and ease of manufacturing integration.
Our goal is to provide the ideal SOLUTION to your requirements.
Visit us at MD&M West 2020 - Booth #2991
