COMPANY INSIGHT
Sponsored by Research Collective
RESEARCH COLLECTIVE
Human Factors and User Experience
Medical devices enable people to live longer and healthier lives. According to regulatory agencies, however, human factors issues are involved in virtually every device related error reported to them. Research Collective, a human factors and user experience company, reduces these errors, making devices useful, usable, desirable and safe.

If a Device Doesn’t Work For People, It Doesn’t Work
Product development organizations have extraordinary resources on hand, including experts in mechanical and electrical engineering, manufacturing, marketing, accounting, and software development. Despite this, the human is the most important component of the system being designed. They are the most variable, the most unpredictable and the most expensive.
Engineers are trained to be experts at technology rather than experts at human behavior. Engineering curricula usually include calculus, differential equations, and physics, not psychology, cognitive science or visual perception. Many engineering programs do not even require a course in introductory psychology. Once employed, engineers spend so much effort on technology that there is little time to think about the device users.
That is where Research Collective comes in. Research Collective applies the same rigor to the device’s user experience as engineers apply to its technology. Human Factors (HF) research and design optimize the interactions between people and the devices they use. It involves observing intended users interacting with a device and measuring various aspects of the experience. This type of work helps uncover the reasons why a user experiences use errors, risks, and difficulties with a device.
As shown in the diagram below, Human Factors is the intersection of social sciences, biological sciences, design and engineering. HF engineers use knowledge of human perception, cognition, emotion, motivation, physical abilities, and social behavior to help clients design usable products. Along the way, the following are continually addressed:
Is the device safe?
Is the device effective?
Is the device efficient?
Is the device easy to use?
Why or why not?
These insights provide a deeper understanding of how the user’s experience can be improved.
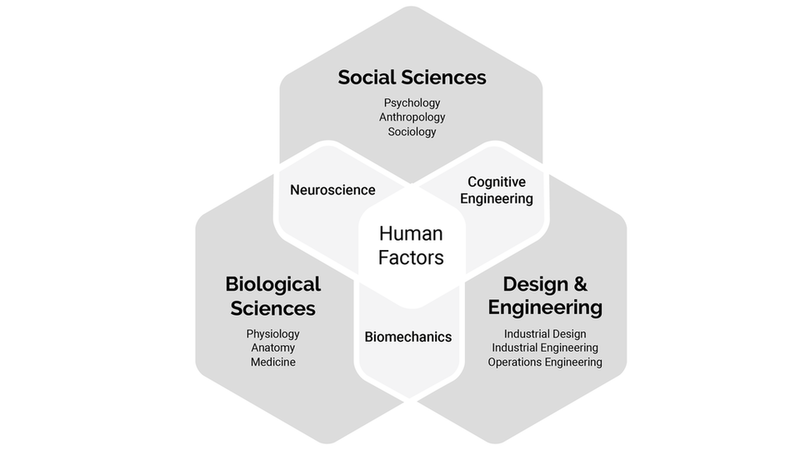
Components of Human Factors
How Can Human Factors Research Improve Products?
Human Factors (HF) research can be implemented at any stage in the product development process. By incorporating HF research early in the design process, products are more likely to be safe, effective, efficient, and easy to use because sources of risk and pain points are identified and remedied early in the development process.
Some devices may be required to undergo Human Factors (HF) usability testing to ensure the device will comply with FDA guidance or EU Medical Device Regulations (MDR). In order for a device to achieve approval or clearance with FDA and a CE mark with the EU, manufacturers must demonstrate that the product can be used safely and effectively by intended users.
HF research methods focus on identifying the root cause, or the reason for, potential sources of harm, which the FDA or notified body reviewers will want to see in the design history file. For example, if a patient monitor alarm for a dangerous arrhythmia does not effectively trigger a nurse’s attention, the root cause may be that the alarm is not salient enough to communicate urgency and/or it does not provide enough information to the user.
Analyzing root causes can be used to develop strong risk mitigation strategies and design modifications that minimize potentially dangerous errors. HF research methods work in an iterative cycle, and when applied effectively, improve safety, efficiency, quality, and reliability, while also reducing long term costs and resources for redesign and risk management.
By implementing Human Factors research into the design process, manufacturers can feel confident that a user can use their device or system safely and effectively.
What Are Some Types of Human Factors Research Methods?
Generative Research: Uncovering User Needs
- Used during the early stages of development to understand current experiences in the market and identify elements of the ideal user experience.
- Examples: Focus Groups, Contextual Inquiry, Participatory Design Research, Card Sorting, etc.
Evaluative Research: Testing an Existing Solution
- Used iteratively throughout the design process to understand whether the design is useful, usable, and desirable.
- An opportunity to identify potential use errors and pain points so they can be mitigated before the design is final.
- Examples: Formative Usability Testing, Design Review, Heuristic Evaluation, etc.
Regulatory: Research Demonstrating the Product is Safe and Effective
- Used at the final stage of development to demonstrate safe and effective use, a detailed report of findings should be documented for FDA or EU notified body reviewers.
- Examples: Formal Validation Study (FDA), Summative Evaluation (EU MDR)
How Can Research Collective Help?
From early-stages of development to market-ready design, Research Collective’s trained Human Factors scientists help manufacturers develop and execute a tailored research plan. The team helps manufacturers produce devices and systems that are:
More likely to meet regulatory requirements
Faster to market
Safer to use
Easier to use
More desirable
Contact information
Research Collective
Web: www.research-collective.com
Email: info@research-collective.com
