Cancer diagnostics using epigenetic markers
Fast, reliable, and non-invasive cancer diagnostics
Scroll down ⇩
Cancer diagnostics
DNA methylation
Pipeline / Contact
GynTect®
Download Flyer: GynTect®
Cancer diagnostics using epigenetic markers
The biotech company oncgnostics focuses on the development of cancer diagnostics. In all tests developed by oncgnostics epigenetic changes, so-called DNA methylation, characteristic for cancer cells are detected. These highly informative markers, identified and validated by oncgnostics, constitute the core of the technology.
First product: GynTect®
The clarification test GynTect® is used in the early detection of cervical cancer and its precursors. Furthermore, the German biotech company develops tests for the medical fields head and neck tumours and ovarian cancer.
DNA METHYLATION ▼
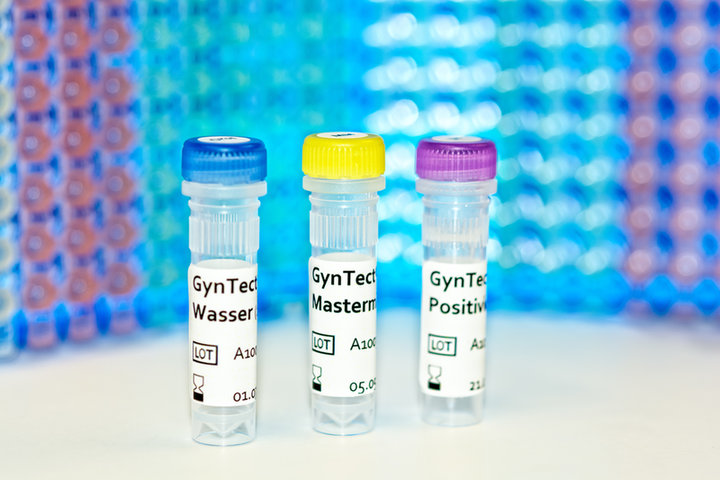
DNA methylation in cervical cancer diagnostics
In the course of cervical carcinogenesis alterations occur in genomic regions. One of them is the hypermethylation of certain DNA regions. The diagnostic test GynTect® allows the detection of DNA hypermethylation of six DNA regions in the human genome specifically occurring in cervical carcinogenesis.
Performance of GynTect® requires a cervical smear. The marker regions are detected by PCR (polymerase chain reaction). The data obtained provide the basis for the GynTect® result. Originally methylated marker DNA regions are amplified in the marker PCRs only. Therefore the method is also called methylation-specific PCR.
PIPELINE / CONTACT ▼


Pipeline: Diagnostics in head & neck tumours and ovarian cancer
Oncgnostics currently validates epigenetic markers for the development of diagnostics for use in other cancers.
Diagnostics available in the field of head and neck tumours are currently very limited. Tumours are only detected when they become macroscopically visible. Therefore the chances of a cure are limited. A test for early detection based on the epigenetic markers validated by oncgnostics could contribute to improve the chances for a full cure. Furthermore, the frequent relapses occurring after removal of a tumour may be detected and treated earlier.
Moreover, oncgnostics works, in close collaboration with the University Hospital Jena, in the development of ovarian cancer diagnostic tests.


Contact:
Oncgnostics GmbH
Winzerlaer Straße 2
07745 Jena
Germany
+49 3641 55485 00
BACK TO TOP ▲