COMPANY
INSIGHT
next generation sequencing device materials
Next Generation Sequencing, so-called “NGS”, is the term coined for the massively parallel sequencing resulting in gigabytes of sequence data per day to quickly identify gene sequences. NGS is being harnessed for everything from personalized medicine to rapidly identifying microbial food contaminants [1]. The best Next Generation Sequencing device materials continue to shift as increasing innovative fabrication and detection approaches to NGS emerge.
Next generation sequencing device materials are complex and manufacture must overcome many hurdles.
Many technologies use optical methods – and in this use case, glass offers the best optical properties. NGS sequencers such as those by Illumina and Pacific Biosystems continue to use glass. Pacific Biosystems is pushing the limits of detection for its new single molecule sequencing product, so background fluorescence and scattering are critical parameters to performance. Emerging next generation sequencing powerhouse, LaserGen, utilizes fluorescence for detection with glass microfluidic flow cells.
Illumina managed to reach the $1,000 genome target by harnessing the power of glass fabrication. By structuring the glass surface, creating patterned flow cells, it was possible for Illumina to increase the density of the sequencing space and reduce image acquisition time since patterned images are easier to overlap during data acquisition rather than unstructured images [2]. A critical parameter for this success is the high accuracy of the sub-micron patterning on a wafer level.
This enables the extremely dense packaging of the sequencing space within the microfluidic chip, reaching the optical resolution limit of conventional fluorescent imaging systems. Details of the patterned flow cells at Illumina, Inc. can be found on the Illumina website [3].
caption
As the $1000 genome gives way to the $100 genome, the desire to minimize costs by reducing the volumes of expensive reagents drives the LOD requirement further down. This increases the need for the low auto-fluorescence of the microfluidic flow cell material. Illumina’s approach relies also on massively parallel analysis, which requires dense feature patterning on glass chips. QIAGEN’s hydrodynamic approach and Bio-Rad’s digital droplet microfluidics design can allow for other materials for the droplet generation that is part of the secret of their sequencing speed [1].
Another cost-reducing approach is to eliminate the label altogether. Companies like Oxford NanoPore and Genia-Roche use a label-free pore-based approach that requires the integration of high aspect ratio nanopores with a direct electronic detection method for sequencing, driving the use of silicon as the microfluidic chip material rather than glass [1]. These new methods relying on CMOS chips for direct electronic detection have also been embraced by Ion Torrent-Thermo Fisher Scientific.
While label-free assays may seem to eliminate the need for the optical properties of glass, glass still offers some advantages. For silicon and glass, the submicron control of the channel etch depth that is possible can result in improved signal-to-noise for impedance detection. The ability to readily incorporate ITO electrodes allows for the integration of digital microfluidic sample preparation, helping to reduce precious sample and expensive reagent consumption.
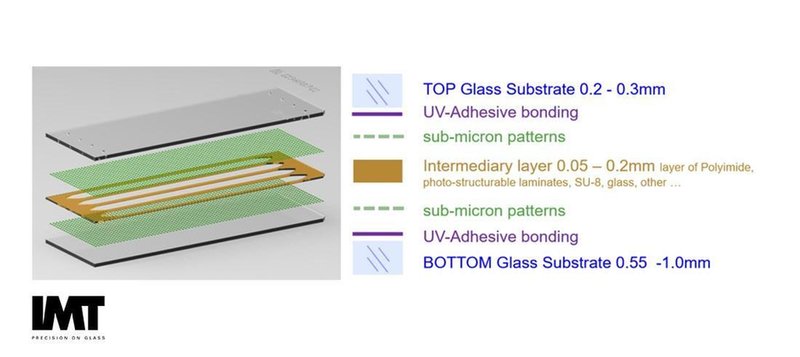
Microscope Slide sized Generic NGS Flow Cell provided by IMT AG. Sub-micron structures are generated on a 200mm glass wafer. This wafer is sealed with a second wafer containing isotropically etched channels, fluidic access holes and an anti-fouling coating; i.e. PEG.

caption
Illumina is a classic example of the in vitro diagnostic life science instrumentation developers in the NGS market. These companies have high competences in the chemistry, meaning they optimize their assay procedures to perform NGS using sequencing by synthesis (SBS); which is where their intellectual property (IP) lies.
Despite the forays into label free solutions, the majority of NGS device developers utilize detection and thus require glass substrates. NGS device development and manufacture requires glass foundries that understand how to overcome the technical challenges of low signal fluorescence detection, often reaching the optical resolution limit of conventional fluorescent imaging systems.
NGS flow cells must be manufactured to custom dimensions, lay-out, and biofunctionalized pattern, with micrometer or sub-micrometer feature sizes, and sealed without disrupting the biofunctionalization. Ideally, the biofunctionalized molecule is an amino-silane alone or on top of a dielectric or metallic coating. The rest of the flow cell channel network is coated by an anti-fouling coating such as polyethylene glycol (PEG).
These complex specifications can be met by a using glass as a primary substrate but require a glass fabrication foundry that can handle the custom binding chemistry; turning the generic platform into a highly customized platform that can be manufactured to scale.

caption
Works Cited
[1] | W. J. Ansorge, "Next Generation DNA Sequencing (II): Techniques, Applications," Next Generat Sequenc & Applic 2016, vol. S1, no. 005, pp. Open Access, dx.doi.org/10.4172/2469-9853.s1-005, 2016. |
[2] | S. Bowen, "Nanotechnology for a Genomic Revolution," in Advanced Technology Workshop on Advanced Packaging for Medical Microelectronics, San Diego, 2017. |
[3] | Illumina, [Online]. Available: www.illumina.com/science/technology/next-generation-sequencing/sequencing-technology/patterned-flow-cells.html. |

caption

