COMPANY
INSIGHT

qmedics
We manufacture state-of-the-art products Environmental-friendly and can flexibly adjust our production capacities to your order situation.
The following are a few of the key medical applications where ElectroCraft motors empower medical motion control.
Company History
QMEDICS is a young Swiss-based medical device manufacturer founded in 2008. Our long year experience in the medical device industry has made us very flexible and highly capable of finding the optimum solution for every customer specific need. We were until 2016 a pure contracted manufacturing company – since then we became a PLM (private label) manufacturing of our own Peripheral product portfolio.
This was a company strategy change that resulted in a new management, new and more R&D engineers, the start of direct customer contact, a sales and marketing strategy in short, a start up in an experienced environment this combination is just ideal.
Our Vision
At QMEDICS We work hard every day. To make sure that our guiding principle strive for state-of-the-art products every day, is reflecting in everything we do.
As a medical device company, we embrace the changing healthcare environment and seek to identify and explore opportunities as they emerge. With the best patient care always in mind, we insist on innovation, highest quality and to put the patient in the middle of our research not economics, profit or politics.
Contracted Manufacturing
Qmedics is a trusted partner with in-house stent and balloon development, manufacturing and logistical service. We supply custom tailored products, solutions and technologies. We produce already for big players in PTA and PTCA medical world.
Our Expertise are the following technologies:
Blow molding
Plastic wielding
Stent loading &
Deployment technologies

Stent & Balloon delivery System & Catheters
Laser cutting and wielding
Surface finishing
Big advantage of Qmedics: We do it all inhouse, no external Processes
R&D
V&V Testing
Quality Compliance

Regulatory Compliance
Manufacturing
Final Packaging


Sterilization

Shipping

Our Portfolio
Pull Stent type
To provide perfect balance between compression resistance and longitudinal conformability, in difficult to cross and moderate to severe calcification lesions
Flex Stent Type
The FLEX property is designed to provide a perfect balance between radial compression resistance and flexion deformation, in complex to cross and moderate calcification lesions
With the tree variants: 4,5,6 Fr compatible introducer.
With the full portfolio of PTA balloons
EXIST 6F
FALCOR 5F
DRACO 4F
Manatee
Naga
Nova
NEWS:
NEW PRODUCT added to our Portfolio
31. July 2019
The EASYX™ Liquid Embolic is a new injectable, precipitating polymeric agent for the obliteration of vascular spaces through direct puncture or catheter access performed under X-ray guidance. The embolic liquid is iodinized Polyvinyl Alcohol (PVA) Polymer ether. Iodine groups are covalently grafted to the PVA polymer backbone, whereby a stable nondegradable polymer with the desired features is created. The resulting polymer is dissolved in Dimethyl Sulfoxide (DMSO).
EASYX™ is CE-marked since December 2016 for the use in peripheral vasculature. The safety and efficacy of EASYX™ embolization liquid for the percutaneous treatment of vascular lesions, i.e. embolization of varicocele, type II endoleaks, portal vein before surgery, active peripheral bleeding or angiomyolipoma (AML) was proven in a clinical trial EASYX-1.
EASYX-1: A Multicenter Study on Safety and Efficacy of Easyx Liquid Embolization Agent Used in Five Separate Indications. Publication is planned very soon.
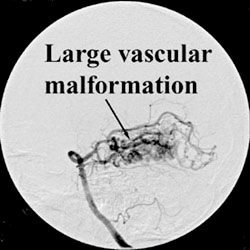
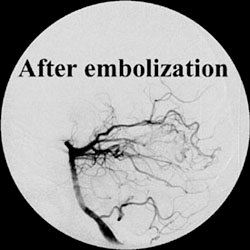
Second clinical trial for the extention of the EASYX indications:
Prospective, Multicenter, Multinational Study to Assess the Safety and Performance of the Easyx Liquid Embolic in Intracranial Interventions IDEALE Study.
This is a Prospective Study to Assess the Safety and Performance of the Easyx Liquid Embolic in Intracranial Interventions.
Applied Harmonics:
These are just a sampling of the applications that ElectroCraft provides motion control solutions for in the medical market, and only a hint at future innovation. As a custom motor and motion control solution provider, ElectroCraft is the ideal partner for your medical equipment requirements.