QMEDICS is a young Swiss-based medical device manufacturer founded in 2008. Our long year experience in the medical device industry has made us very flexible and highly capable of finding the optimum solution for every customer specific needs.
Ever since our foundation, we have been based in North-East of Switzerland, a region famous for technical innovations, with an international character and a sense of global trading. At the beginning of QMEDICS’ existence, QMEDICS offered contract R&D, manufacturing as well as OEM manufacturing for medical devices. As OEM manufacturer we developed, produced and supplied a workhorse of a Nitinol stent QM1 with the matching balloon catheter. This was the pride of the company and became the base of the new product portfolio of today.
In 2017 QMEDICS became an independent company with the opportunity to be legal manufacturer of our own new product portfolio and to do direct sales.
Since the know-how, engineers and production of this design are still at QMEDICS we could improve and expand our product portfolio within a minimum of time.
Today the total facility has an area of over 2800m2 of production space, storage areas and offices, including two clean rooms with a total surface of 1200m2. The facility houses sophisticated machinery, EtO-sterilization as well as packaging and labelling equipment. Finally, dedicated, motivated and highly experienced employees.
Even the best strategy can’t succeed unless it’s supported by a strong company spirit. That’s why we at QMEDICS live and foster an Ownership Culture – a culture that encourages every individual in our company to give his or her best in his or her position to help build QMEDICS’ longterm successes.
Our Vision
At QMEDICS we work hard every day to make sure that our guiding principle “striving for excellence every day and constantly seeking to improve the services we offer” is reflected in everything we do.
QMEDICS as a provider of special patient-care products within the medical device industry, we offer global solutions to improve the quality of life
As a medical device company, we embrace the changing healthcare environment and seek to identify and explore opportunities as they emerge. With the best patient care always in mind, we insist on the utmost in quality of products, character of employees and relationships with customers and colleagues. We commit to strengthen our position in the market and being a financially viable company through innovation and continuous improvement
Our Mission
Understanding your business is our business
Our goal is to design, develop, manufacture, and sell state-of-the-art medical products that will improve the lives of patients around the world.
Partnership instead of competition
We manufacture state-of-the-art products, environmentally-friendly and can flexibly adjust our production capacities to your order situation.
Sharing Expertise is our mission and a promise to customers and colleagues to share medical knowledge and expertise for the benefit of patients health.
Our experience and Swiss quality is your guarantee
We are a community and we serve a community. Nothing we do stands alone, everything depends on cooperation and collaboration.
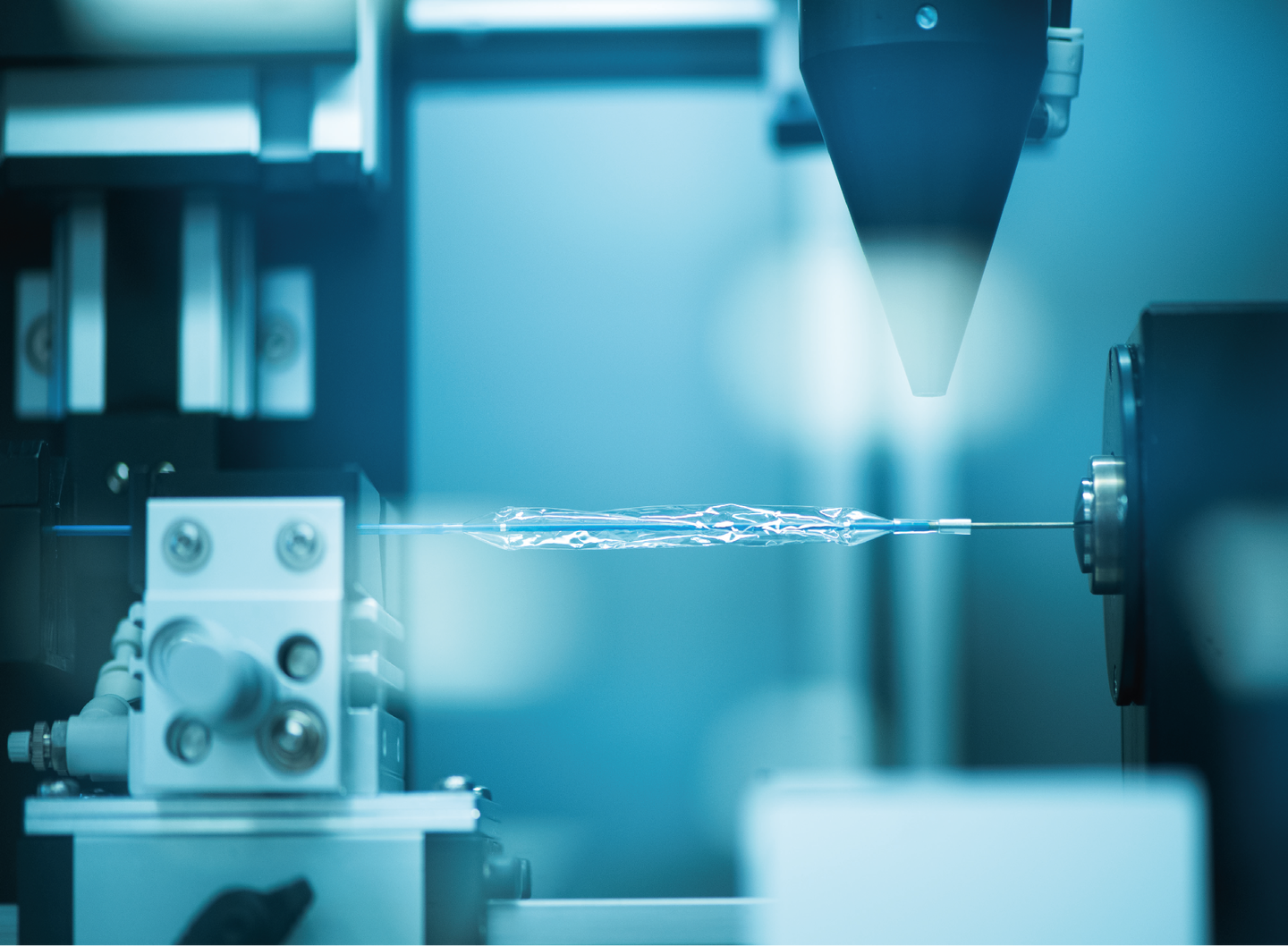
- QMEDICs was founded in 2008 out of a SWISS holding with different companies, the responsibility within this holding was OEM & Contracted manufacturing with R&D services. QMEDICS’ R&D developed, produced and supplied a workhorse of a Nitinol stent QM1 with the matching balloon catheter.
- The core Technology, Stent & Balloon Catheter manufacturing.
- Material expertise : NiTi, CoCr, Stainless Steel & Tantalum, Poly Amide, Poly Imide, PEEK,PEBA, PC, PUR & PTFE
- In 2017 QMEDICS became an independent company under the Swiss TF holding, which gave us the opportunity to be legal manufacturer of our own new product portfolio and to do direct sales.
- Since the know-how, engineers and production teams of the QM1 stent system are still at QMEDICS we could improve and expand our product portfolio within a minimum of time.
- Our years of experience, with production for other companies gives us a broader view on strategic product development and a reliable production. This experience we now implement into our own Product portfolio.
- Today the total facility has an area of over 2800m2 of production space, 1000 m2 storage areas and offices & two clean rooms with a total surface of 1200m2.
Production Teams
Our manufacturing personal is dedicated, motivated and highly experienced. They play a vital role in the positive experience and healing of our patients and in the success of our company.
Serving the needs of others is the reason why they work every day with the same enthusiasm trustworthiness creativity and discipline with the result of producing state of the art products


Stent Manufacturing Team




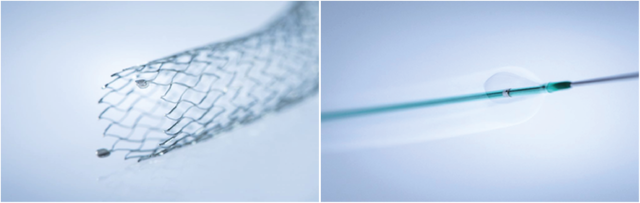
Draco 418 – 418 Nova
Falcor 518 – 518 Naga
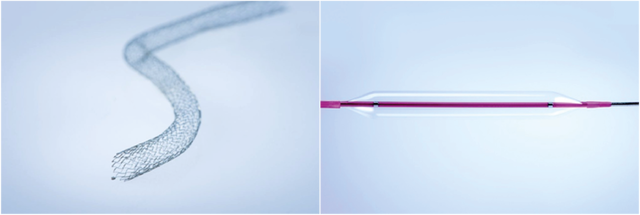
Exist 635 – 635 Manatee

Big advantage of Qmedics: We do it all inhouse, no external Processes
R&D
V&V Testing
Quality Compliance

Regulatory Compliance
Manufacturing
Final Packaging


Sterilization

Shipping