COMPANY
INSIGHT

Biocompatible Coatings for the Evolving Medical Device
BioInteractions has dedicated over twenty-five years into researching and developing innovative, biocompatible solutions for the challenges faced by medical devices today. Our commitment of advancing healthcare through innovation, has resulted in our range of proprietary biocompatible coatings to target the specific issues of thrombus formation, device related infections and tissue inflammation. Our coatings enable medical devices to perform their intended function as well as reduce patient complications throughout their therapy.
The following are a few of the key medical applications where ElectroCraft motors empower medical motion control.
Commitment to Care
BioInteractions has maintained a position at the forefront of the ongoing quest for more sophisticated biocompatible materials, supporting our partners in providing breakthrough solutions to the medical device industry.
All medical devices face common challenges when required to perform their function inside the body. These biocompatible challenges are generally seen in the areas of, thrombus formation, device related infections and tissue inflammation at the device-body interface. BioInteractions has created a range of non-leaching, biocompatible coatings which utilise active components to confront these challenges. This active approach supported by a passive process is a distinct advantage offered by our technology.
Our expertise in the medical device industry has helped us to understand the level of importance in applying the polymer coating as well as the polymer coating itself. We have found that the efficacy of the coating may also be significantly impacted by its process of application onto the device. Our know-how in these areas allows us to provide both the polymer coating and the coating services for our customers.
BioInteractions are able to supply our coatings as well as coat the devices in our cleanroom facility.
Astute®
Triple-Endothelial Action
Anti-thrombogenic Coating
The response observed when a foreign material comes into contact with blood can be very aggressive and has the potential to cause multiple complications for the medical device and impact patient well-being. The foreign material response includes protein deposition and platelet activation which leads to thrombus formation.
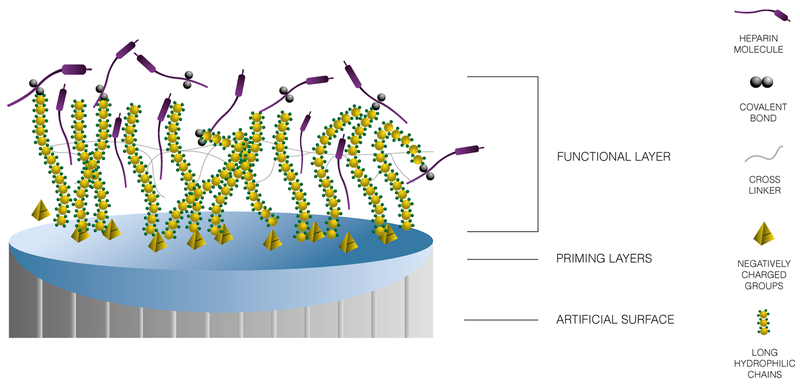
BioInteractions has functionalised heparin into a polymer in order to actively reduce the foreign material response. The functionalised heparin combined with a negative charge and a physical repulsion provides an active triple endothelial-like layer which actively prevents thrombus formation.
Triple Endothelial-Like Action:
Non-leaching Heparin
Heparin is cross-linked onto the surface to actively prevent thrombus formation and provide similar benefits as heparin sulphate found in natural endothelium.
Negatively charged groups
Strong negative charge repels blood cells and proteins from the device surface.
Highly hydrophilic chains
Physically minimises the interaction between blood elements and the coating.
Schematic representation of Astute®
Astute® Antithrombogenic Coating takes an active approach to preventing thrombus formation. The non-leaching heparin combined with negatively charged groups and the physical interaction of the hydrophilic chains provide a multi-faceted approach to prevent thrombus formation and device occlusion which provides distinctive benefits from the coating:
Negates platelet activation and reduces the risk of blood from clotting further downstream.
Promotes laminar flow which helps to improve the function of devices as well as reducing protein and cellular deposition.
Non-leaching coating which provides long-term efficacy throughout the lifetime of the coating.
Astute® Antithrombogenic Coating has been FDA approved and has aided in maintaining the competitive position of our customers’ devices in their chosen industry for over fifteen years.
AvertPlus™
Dual Active Contact Kill Mechanism
Antimicrobial Coating
The majority of medical devices face a common issue of device related infections as they are increasingly utilised in the therapy of patients. These device related infections can lead to further complications in the patient’s therapy, and in turn potentially lead to further risks and complications.
The main challenge is the adhesion of bacteria onto the device surface or onto existing proteins that are bound to the device surface. This provides a thriving environment for bacterial colonisation and biofilm formation. Bacteria within biofilm are 50-500 times less susceptible to antibiotics and are able to migrate through the bloodstream to spread infection.
BioInteractions have developed a coating with a two factor approach to actively tackle bacteria deposition and biofilm formation on the device surface. The active and passive components of the polymer coating provide an effective method to inhibit protein deposition and bacterial adhesion to prevent biofilm formation.
Dual Active Contact Kill Mechanism
Non-leaching active antimicrobial agent
Polarised antimicrobial agent interacts with the bacteria at multiple points which induces separation of the cellular membrane causing the cell to lyse.
Highly hydrophilic, non-thrombogenic component
Long hydrophilic chains, physically prevent protein deposition and bacterial adhesion onto the device surface.
Schematic representation of AvertPlus™ Surface Active Antimicrobial Coating
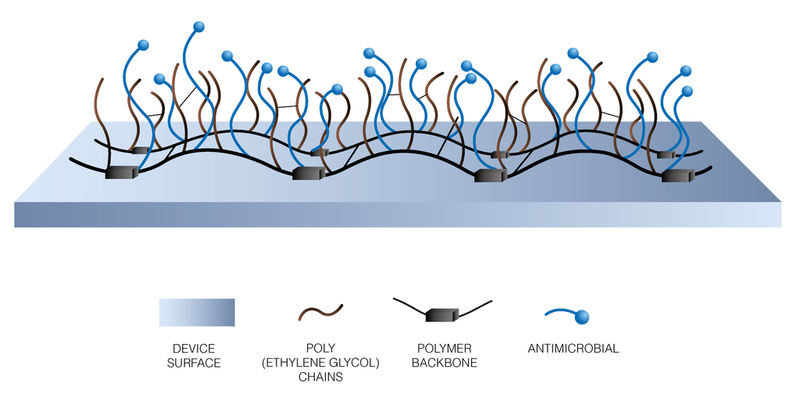
AvertPlus™ Antimicrobial Coating utilises the dual functionality of an active agent combined with a passive component to prevent bacterial colonisation and biofilm formation. This two factor approach to tackling the challenge of device related infections gives the coating unique benefits:
Targeted active component that prevent only bacteria from binding to the surface.
Broad spectrum of activity across a variety of microorganisms including, gram positive, gram negative and fungi.
Non-leaching components that give long-term efficacy throughout lifetime of coating.
AvertPlus™ Antimicrobial Coating has been CE marked and has been used on our customers’ devices for a number of years.
Assist™
Enhanced Lubricious Activity
Hydrophilic Coating

An increase in the use of therapies which are delivered through the body pathways has met significant challenges at the device body interface. These challenges exist due to the frictional forces between the device surface and the surrounding tissue.
These frictional force restrictions can lead to further complications for the patient. These may include an increased risk of tissue damage, prolonged procedures and increased levels of patient discomfort.
BioInteractions has tackled these challenges with a dual factor solution that improves the lubricity of the surface and reduces the thrombogenic activity around the device-body interface. Our hydrophilic technology offers a simple coating application process and has the ability to be used with a UV curing or Heat Curing method.
Enhanced Lubricious Activity
Highly lubricious component
Improves the friction coefficient of the surface and reduces the restriction between the device and body interface.
Non-thrombogenic component
Prevents deposition of cells and proteins onto the device surface
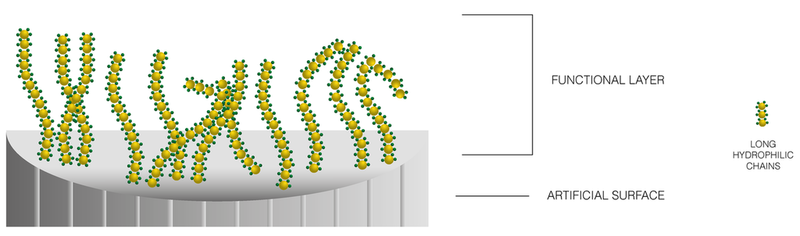
Assist™ Hydrophilic Coating provides two factor protection against the challenges faced on a device surface. The combination of these factors being present in the coating gives advantages that can be felt by both the clinician using the device and the patient receiving therapy from the device. These benefits are seen by:
Reduced patient discomfort
Enhanced delivery and effortless removal of the device
Assist™ Hydrophilic Coating has been FDA approved and utilised in the medical device market around the world.

