Wearables
NightWare vs nightmares: the sleep tech app helping break PTSD patterns
Many people with post-traumatic stress disorder experience nightmare disorder. Now, NightWare has utilised the Apple smart watch to create a platform designed to stop these nightmares, without waking the wearer. Chloe Kent speaks to NightWare CEO Grady Hannah to learn more.
D
reams are an enduring mystery for scientists and psychologists. While researchers have come to understand the neuroscience behind what happens in our brains when we dream, exactly why the process occurs still isn’t completely clear.
For most people, dreams are merely banal or bizarre in nature. For others, they can take on a terrifying nature and become highly disruptive to their quality of life. While frequent nightmares are most common in children, especially those between the ages of three and six, adults with conditions like post-traumatic stress disorder (PTSD) can find themselves experiencing regular nightmares that can be incredibly difficult to predict or prevent.
It’s thought that 50% to 75% of people with PTSD have nightmare disorder, disruptive nightmares that happen about once a week.
“These are not the type of nightmares that someone without PTSD might have,” says NightWare CEO Grady Hannah. “These are re-experiencing of the trauma that led to the PTSD, or some allegory of it, and it’s an experience that inevitably wakes the sleeper up.”
While talking therapies and off-label pharmaceuticals have been used to treat nightmare disorder in the past, NightWare has now developed an artificial intelligence (AI)-powered digital therapeutic that it claims is the first and only medical device designed to reduce nightmare-related sleep disturbance in adults.
NightWare uses an Apple watch and an iPhone that are configured and logged into both a software application and the NightWare server, to help interrupt the wearer’s nightmare without waking them up – giving them a better chance of an undisturbed night’s sleep.
The platform received breakthrough device designation from the US Food and Drug Administration (FDA) in late 2020.
The science of dreams
Sleep can be divided into two basic types: rapid eye movement (REM) and non-REM sleep, which has a few different stages. A person cycles through all stages of non-REM and REM sleep several times over the course of a night, with increasingly longer and deeper periods of REM occurring towards the morning.
In stage one of sleep, the heartbeat, breathing and eye movements slow and the muscles relax, as the brain begins to slow down and transition from wakefulness to sleep. In stage two, eye movement completely stops and brain waves become slower, with occasional rapid bursts of activity known as sleep spindles – this stage forms 45% to 55% of total sleep time.
REM makes up about 20% of our time asleep, and it is in this stage that vivid dreams and nightmares occur most often.
In stage three, extremely slow brain waves called delta waves begin to appear, interspersed with smaller and faster waves, while in stage four the brain produces delta waves almost exclusively.
No eye movement or muscle activity occurs during stage three and four and waking someone in these sleep stages can be difficult and leave them feeling disoriented.
Stage five is where REM occurs. Breathing becomes more rapid, irregular and shallow and the eyes jerk rapidly while limb muscles become temporarily paralysed. REM makes up about 20% of our time asleep, and it is in this stage that vivid dreams and nightmares occur most often.

NightWare uses an Apple watch and an iPhone that are configured and logged into both a software application and the NightWare server. Credit: NightWare
How does NightWare work?
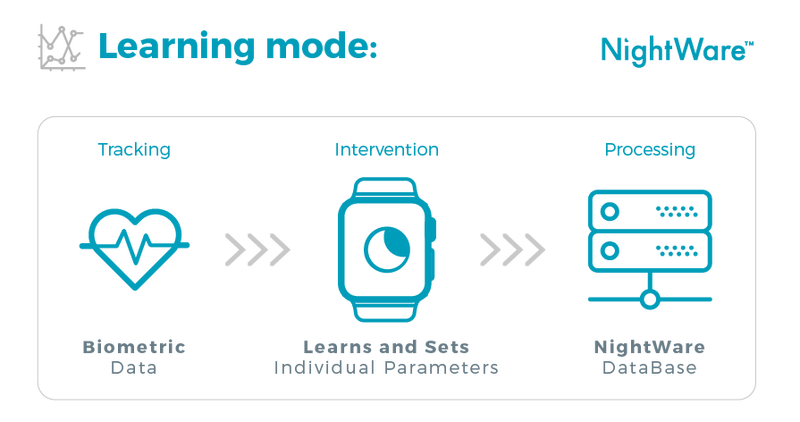
To target the excessive nightmares of people with PTSD, NightWare continuously collects heart rate, accelerometer and gyroscope data from the wearer using the pre-existing technology inside the Apple watch. The AI in the app uses this data to compute a stress threshold for each individual user, defined by their heart rate and movement. NightWare learns the wearer's sleep pattern after just a few days of wearing the watch each night, typically less than ten.
If the wearer’s heart rate begins to rise and they begin to move more rapidly, the app triggers the watch to vibrate. The vibration starts at a low intensity, which increases if the wearer’s heart rate and movement continue above their stress threshold. The goal of the vibration is to arouse the user, without fully awakening them.
If the wearer’s heart rate begins to rise and they begin to move more rapidly, the app triggers the watch to vibrate.
“In certain cases, the watch may actually wake someone up,” says Hannah. “But when the nightmare hasn’t gotten to completion, people are more readily able to fall back to sleep because their cortisol levels aren’t up, along with all the other bodily reactions that happen when you have a completed nightmare.”
If the watch senses that it woke the wearer, it will vibrate less next time to try and avoid this. Users wear the watch only while sleeping, and not during the day, as this is when it recharges. Wearing it during the day can also risk triggering a false alert if the wearer is doing something like reading or watching television, as these activities can cause the heart rate to spike.
NightWare continuously collects heart rate, accelerometer and gyroscope data from the wearer. Credit: NightWare
Utilising Apple’s innovation
The platform is currently available in the US on a limited basis through physician recommendation. It ships with the Apple watch and iPhone as part of a package, meaning users don’t have to incorporate their NightWare treatment into their personal devices.
“However big of a company we were, we were not going to have the same R&D budget as the Apple watch,” says Hannah. “As Apple continues to innovate, we’ll be able to benefit from that as well. It’s got two FDA clearances and it has run multiple clinical studies on its EKG and atrial fibrillation capabilities.”
As Apple continues to innovate, we’ll be able to benefit from that.
NightWare was studied in a 30-day sham-controlled trial of 70 patients before it received its recent FDA approval. Patients in both groups showed improvement on two versions of the Pittsburgh Sleep Quality Index scale, with the active group showing greater improvements than the sham group.
Hannah says: “Running a randomised, placebo-controlled, double-blind clinical trial was an important decision. Through being able to make claims, have regulatory clearance and be published, prescribers will feel more comfortable to recommend and build treatment plans around NightWare.”
A holistic approach to PTSD
The firm is currently focusing on working with the Veterans Health Administration (VHA) health care system and the Department of Defense Military Health System in order to reach veterans, who are more likely to experience PTSD than civilians. Around a third of patients accessing mental health care through the VHA have PTSD.
Ultimately, NightWare is a treatment that seems most appropriately used as part of a holistic healthcare plan for PTSD. In its statement about the approval of the device, the FDA said that: “NightWare is not a standalone therapy for PTSD. The device should be used in conjunction with prescribed medications for PTSD and other recommended therapies for PTSD-associated nightmares and nightmare disorder.”
Around a third of patients accessing mental health care through the VHA have PTSD
Hannah says: “PTSD is a persistent and hard to treat problem. In the case of some cognitive behavioural therapy exercises like prolonged exposure, where a person with PTSD is asked to relive their experience, it can lead to heightened nightmares.
“There may be a balance where utilising NightWare during some of these more intensive therapies may help the user get through better, so that they can experience all the cathartic aspects without showing up in the morning having not had any sleep the previous night.
“The only pharmaceutical treatments for nightmare disorder are off-label, and some of them have pretty severe side effects. I think if you give people the opportunity for treatment with drugs or no drugs, intuitively they might choose the no drug option. But we’re not here to dictate to anybody what their care plan should be. Our focus has been on staying in our lane and solving this one narrow but difficult problem.”