Cover story
Alzheimer’s: drug delivery and the blood-brain barrier conundrum
The blood-brain barrier is the biggest hurdle in treating brain diseases, and research on overcoming this challenge is ongoing, writes Ross Law.

The blood-brain barrier (BBB) is formed by microvascular endothelial cells lining the cerebral capillaries penetrating the brain and spinal cord. Credit: Butusova Elena / Shutterstock
With the 2024 edition of the Alzheimer’s Association International Conference (AAIC) having recently taken place in Philadelphia, US, research into approaches to treating Alzheimer’s disease continues at pace. And it is no surprise, given the number of people living with the condition continues to rise and few drugs are available on the market for treatment.
Alzheimer’s is the most common form of dementia. Alzheimer’s Disease International (ADI) estimates there were more than 55 million people worldwide living with dementia in 2020 and anticipates this number will almost double every 20 years, reaching 78 million in 2030 and 139 million in 2050.
Earlier this month, the US Food and Drug Administration (FDA) granted market approval for Eli Lilly’s monoclonal antibody (mAb) donanemab (Kisunla).
It joins lecanemab (Leqembi), which was approved by the FDA last year, as the second disease-modifying therapy (DMT) to come to market in the US that is a mAb designed to target and reduce the build-up of amyloid-beta plaques in the brain. The build-up of this protein is a key characteristic implicated in neurodegenerative diseases.
GlobalData analysts forecast that sales of lecanemab and donanemab could generate global sales of $3.5bn and $2bn respectively by 2030.
While the Alzheimer’s drugs on the market display encouraging signs of efficacy, with donanemab granted FDA approval based on data showing it achieved a 35% slowing of cognitive decline in patients after 18 months, the journey towards treating the disease is far from over.
Past research has shown that the blood-brain barrier (BBB), a natural filtration system of the brain, limits the penetration of more than 98% of drugs.
The BBB therefore also presents a key challenge to the use of antibodies for treatment, since only a small proportion of the administered drug is able to enter the brain via an intravenous (IV) delivery method.
This restriction generally means that surpassing the BBB requires higher doses of a drug, and more frequent treatments when delivered IV. However, higher doses of medications can create toxicity or side effects for the rest of the body.
The use of mAB treatments for Alzheimer’s also holds potential safety concerns, namely in amyloid-related imaging abnormalities (ARIA). The side effects for some patients, perceivable in brain imaging, can result in swelling and potential bleeding in the brain.
Past research has shown that lecanemab caused ARIA with oedema or effusions in 12.6% of a 698-participant group.
On 25 July, the European Medicines Agency (EMA) refused marketing authorisation for lecanemab, citing its belief that the benefits of the treatment were not large enough to outweigh the ARIA risks.
Taken together, these factors are the reason that research is underway in addressing Alzheimer’s with alternate drug delivery (DD) methods, aimed at penetrating the BBB more effectively or circumventing it.
Delivering stem cells into the brain’s ventricles
Regeneration Biomedical CEO Christopher Duma is thrilled that mAB treatments for Alzheimer’s are now available on the market, but he is not convinced amyloid-targeting alone is the ideal approach to treating Alzheimer’s.
“So many things are involved in Alzheimer’s. There’s oxidative stress, mitochondrial dysfunction, autophagy problems, and neuroinflammation – all of these things can’t be solved with a ‘single bullet’,” he says.
Therefore, Duma believes that in addressing Alzheimer’s, something with pluripotent potential is needed.
“There’s only one thing that can do that, and that’s a stem cell,” he says.
Mitigating the challenge presented by the BBB, since it does not involve going through the blood, Regeneration’s DD approach for Alzheimer’s is in delivering RB-ADSC, its autologous stem cell treatment, through an Ommaya reservoir – a type of ventricular catheter — into the cerebrospinal fluid (CSF) within the brain’s porous ventricles.
Ventricles are involved in the creation of the CSF that circulates throughout the brain and spinal cord, serving as a conduit in ‘washing out’ debris in the brain – a process that occurs daily as we sleep.
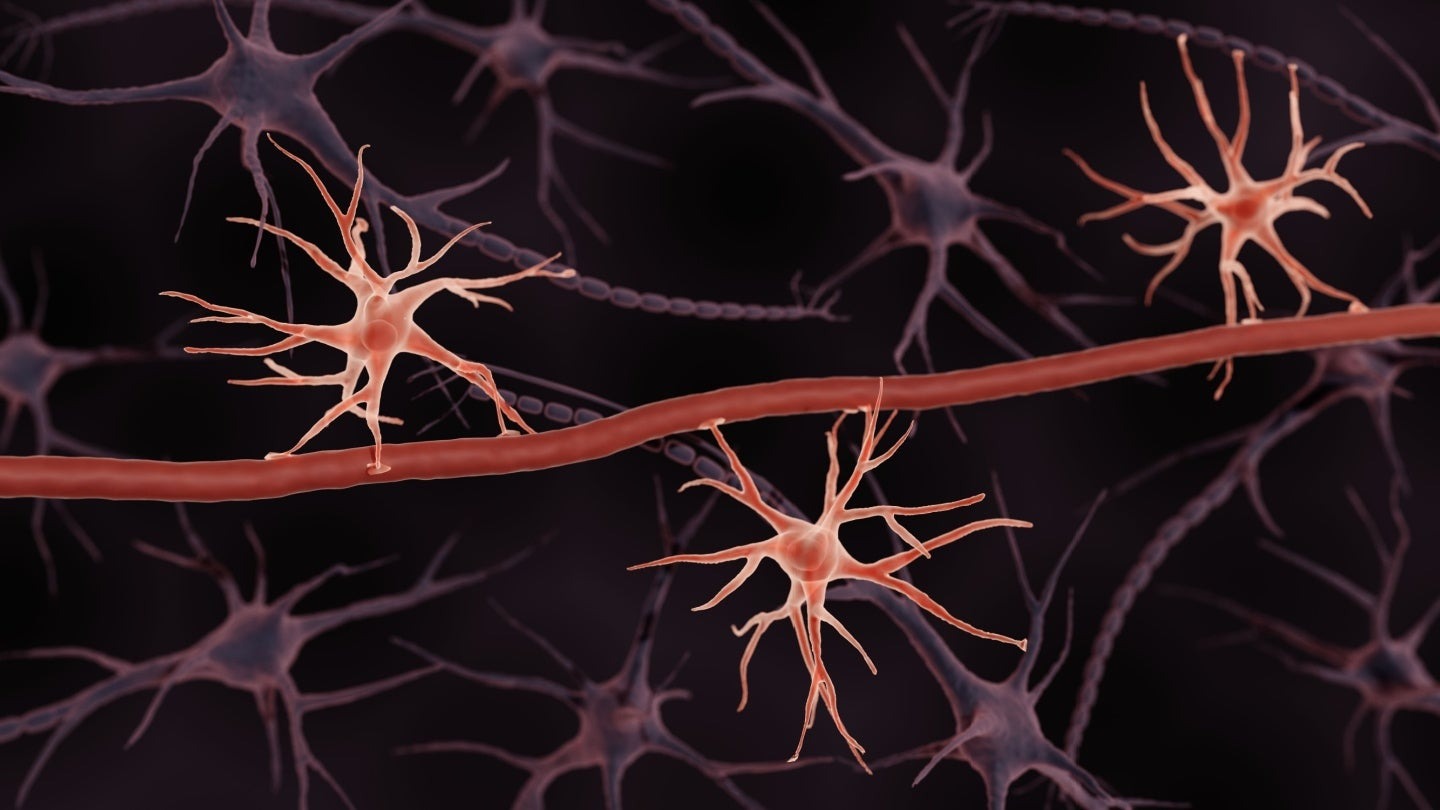
Astrocytes are a type of neural cell that builds the BBB, and Excellio plans to derive exosomes from them to make them even better at targeting the brain. Credit: ART-ur / Shutterstock
Duma points out that the likes of amyloid-beta and phosphorylated tau (P-tau) are normal constituents of the brain, but they run amok in Alzheimer’s due to a compromised immune system, potentially due to inflammation.
The theory is that stem cell delivery for Alzheimer’s may support immune system repair and help improve the washout of beta amyloid and P-tau.
In addition, Duma hopes that injecting stem cells will harness stem cells that already exist in the brain but are not being utilised in brain repair.
“By virtue of the reservoir being in, we believe our stem cells are getting through the porous openings of the walls of the ventricles and stimulating the stem cells that are living in the walls of the ventricles,” Duma says.
“What we’re hoping is that the layers of the ventricles allow the stem cells to go through and stimulate other stem cells and then start their cascade of repairing and replacing damaged brain tissue.”
In April 2024, Regeneration enrolled the first patients in a Phase I trial of its treatment, of which the primary endpoint will be to evaluate safety. Phase II would see the company injecting patients with their stem cells to evaluate the theoretical effect stem cells could have on areas such as immune system repair.
Initial findings from Regeneration’s Phase I study were shared in a poster presentation at AAIC.
Delivering drugs to the brain with exosomes
Scotland-based Excellio is focused on enhancing approaches to DD given that the systemic delivery (IV) of drugs can cause toxicity.
The company has learned that therapeutics can be placed into exosomes – vesicles that act as messengers between cells and carry genetic information and proteins – for their use as delivery vehicles.
Excellio intends to maximise exosomes’ efficiency as DD vehicles by deriving them from specific cells and making them tissue specific.
We will put certain types of markers on them that, like a GPS, will steer the exosome towards the tissue that we want to target
Excellio CEO and co-founder Kasia Maj
“Exosomes are small enough to pass through the BBB,” adds Excellio CSO and co-founder Kamila Malysz.
“They also have that natural affinity to the cell of origin, so if you derive them from neural tissues, then they will specifically target neural cells.”
According to Maj, astrocytes are particularly good candidates for surpassing the BBB.
“Astrocytes are a type of neural cell that build the BBB, so if you derived exosomes from those astrocytes, they’re even better at targeting the brain,” she explains.
Excellio also plans to enhance exosomes’ DD potential by engineering them to be more specific.
“Adding a peptide from the rabies virus to the membrane of exosomes makes them much more specific to the brain, and adding other peptides, which we’ll be working on when we characterise our exosomes, may further increase their efficiency in brain targeting,” says Maj.
Alzheimer’s and gene therapy: the intra-cisterna magna (ICM) route
Passage Bio’s lead candidate PBFT02 is an adeno-associated virus vector 1 (AAV1) capsid-based gene therapy.
“The premise of this gene therapy is that it encodes for a protein called progranulin (PGRN),” says Passage Bio CEO Will Chou.
The company is currently evaluating PBFT02 for treating frontotemporal dementia (FTD) with mutations in the granulin (GRN) gene but plans to move into Alzheimer’s in future as it is another indication in which PGRN deficiency has been implicated.
PBFT02 offers a functional copy of the GRN gene in people with mutations in the gene encoding progranulin.
“There is a good amount of epidemiological and pre-clinical evidence that low levels of PGRN can worsen the phenotype of Alzheimer’s disease, and high levels of PGRN can improve it,” says Chou.
“That’s why we will be targeting Alzheimer’s patients who have a single nucleotide polymorphism (SNP) in their GRN gene. Patients who have this SNP tend on average to have lower levels of PGRN.”

The Rockefeller Neuroscience Institute has been evaluating the use of focused ultrasound (FUS) to temporarily open the BBB. Credit: mapush / Shutterstock
PBFT-02 is delivered intra-cisterna magna (ICM), which involves injecting an established dose of therapeutic virus into the CSF-filled subarachnoid space via the cisterna magna.
Administration takes around 45 minutes and is done via computed tomography (CT) guidance. And the BBB is not a mitigating factor with this DD method as it is penetrated by the administrating needle.
“What that means is that we can deliver much lower doses of vector than a systemically administered product, which has real consequences on the product’s safety,” says Chou.
While some gene therapies delivered systemically necessitate screening patients for neutralising antibodies which could knock out the effect of an AAV capsid, Chou says this potential complication is overcome with DD into the CSF, given it is a relatively immune-protected space and does not contain any circulating neutralising antibodies.
Used in the same way for Alzheimer’s as it is currently being evaluated for FTD, PBFT02 aims to affect the proximal root cause of these patient’s neurodegeneration.
“By replacing the progranulin, you’re really addressing the root cause,” says Chou.
“The newly approved products for Alzheimer’s are affecting a physiology that is well correlated with clinical decline, but it’s not necessarily the root cause of why people have the disease.”
Opening the BBB with focused ultrasound
Dr Ali Rezai, director of the WVU Rockefeller Neuroscience Institute, has been evaluating the use of focused ultrasound (FUS) to temporarily open the BBB.
FUS in combination with mABs is intended to enable higher doses of antibodies (the institute is evaluating this process with lecanemab) to reach the brain quicker than mAb alone.
“Since Alzheimer’s is a progressive disease, the potential for a faster clearing and removal of the beta amyloid plaques in a few months is of importance versus waiting for 18-24 months of biweekly infusions,” Rezai says.
He views some other approaches to overcoming the BBB as high risk and invasive. In contrast, he notes that FUS is a non-surgical, minimally invasive, and outpatient approach that can, in a highly focused and targeted fashion, be used to open the BBB.
FUS directed at a specific region of the brain works alongside an injection of microbubbles to disrupt the BBB.
“These microbubbles travel to the brain through the blood vessels, and when the FUS is precisely delivered and hits the bubbles, they expand and constrict,” explains Rezai.
In doing so, they stretch the vessels just enough to open the BBB.
The Rockefeller Neuroscience Institute’s first endpoint in its research will be in determining if it can safely combine lecanemab treatment with FUS BBB opening in specific brain regions with high beta-amyloid plaques detected with PET scans, with the secondary endpoint to determine if combining FUS BBB opening with lecanemab clears amyloid faster than lecanemab treatment alone.
“If these two endpoints are met, the overall goal is to safely open up larger areas of the BBB so that we can more rapidly clear a clinically meaningful amount of amyloid and investigate the clinical benefits of this combination therapy,” says Rezai.
While amyloid plaque build-ups are characteristic of Alzheimer’s and neurodegenerative decline, there appears to be a move towards exploring the potential root causes of the condition in more detail with other treatment approaches.
The evaluation of DD methods that allow for increased uptake of drugs by the brain, which are not limited by the BBB, comprise an important part of current Alzheimer’s drug development.
Unifying the right kinds of drugs with the right treatment and DD method, may in time break new ground in Alzheimer’s treatment, with research currently underway suggesting that the IV DD of mAB and their function in treating Alzheimer’s, may only be scratching the surface around treatment efficacy and approaches that may be seen in future.
Caption. Credit:

Phillip Day. Credit: Scotgold Resources
Total annual production
Australia could be one of the main beneficiaries of this dramatic increase in demand, where private companies and local governments alike are eager to expand the country’s nascent rare earths production. In 2021, Australia produced the fourth-most rare earths in the world. It’s total annual production of 19,958 tonnes remains significantly less than the mammoth 152,407 tonnes produced by China, but a dramatic improvement over the 1,995 tonnes produced domestically in 2011.
The dominance of China in the rare earths space has also encouraged other countries, notably the US, to look further afield for rare earth deposits to diversify their supply of the increasingly vital minerals. With the US eager to ringfence rare earth production within its allies as part of the Inflation Reduction Act, including potentially allowing the Department of Defense to invest in Australian rare earths, there could be an unexpected windfall for Australian rare earths producers.
