NSF International works with in vitro diagnostic companies of all sizes, providing customized end-to-end services throughout the product lifecycle.
NEW IVD APP!
NSF has launched a brand new IVD app to help companies prepare for IVD regulation (EU) 2017/746. Looking to get the latest IVD industry news and regulatory updates straight to your device? Look no further! Available to download today for free on Apple’s App Store and Android’s Google Play.

IVD APP FEATURES
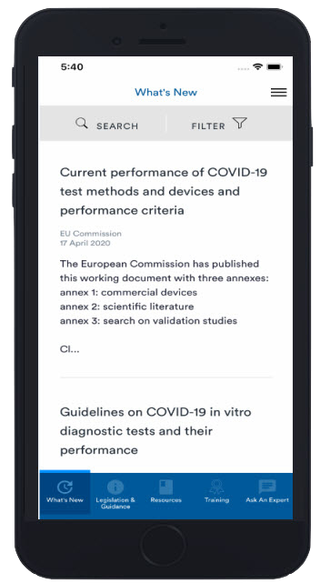
What’s New?
Stay up to date with the latest IVD regulatory updates as well as other key medical device news – get notifications straight to your device!
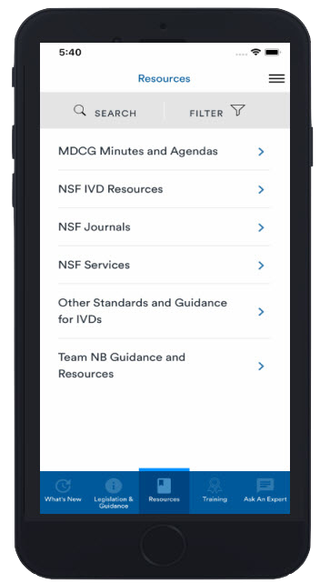
Resources
From white papers and webinars to NSF Journals, our resources section holds a wealth of assets you can access for free. Listen, read or watch straight from your device!

Ask an Expert
Have an urgent question or need quick advice from an expert with years of industry knowledge? We have you covered with our Ask an Expert feature. With one click, you can ask a question to one of our many NSF industry experts and receive a response within 48 hours.
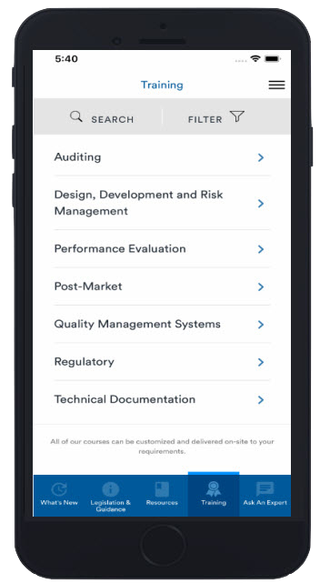
Training
Access our range of training information, book onto courses or enquire about our in-house and virtual training which can be tailored to meet your organization needs. Our IVD app also houses all of our eLearning courses, which can be accessed through your device and completed at a time and place to suit you.
Our IVD Services
From concept through to distribution, our expert services enable:
- Efficient clinical trial conduct
- Risk appropriate regulatory compliance
- Implementation of effective and lean quality management systems
- Maximized contributions of existing staff
- The highest levels of product quality and safety
- Improved competitive advantage and speed to market
Our diverse team allows us to combine global regulatory knowledge with industry best practices to provide you with customized technical, quality, compliance and regulatory services.
For more information about the services we offer to the medical device industry visit our website or contact us.


