Incorporation of Human Factors into the
Medical Device Product Lifecycle
Getting a medical device into the market is not an easy process and so medical device manufacturers have committed to following some type of defined Product Lifecycle. The basic flow starts from the early stage of identification of an idea through to the commercialization of the that idea. There are a number of sub-processes and checks and balances (e.g., gates) that followed during each of these phases. Importantly, it has been shown that incorporation of Human Factors practices into these sub processes assists in getting a safe, effective, and profitable product into the hands of the intended user for the intended purpose in the intended environment. Regulatory and notified bodies have come to realize the importance of Human Factors integration into the Product Lifecycle and have made it a requirement/regulation.
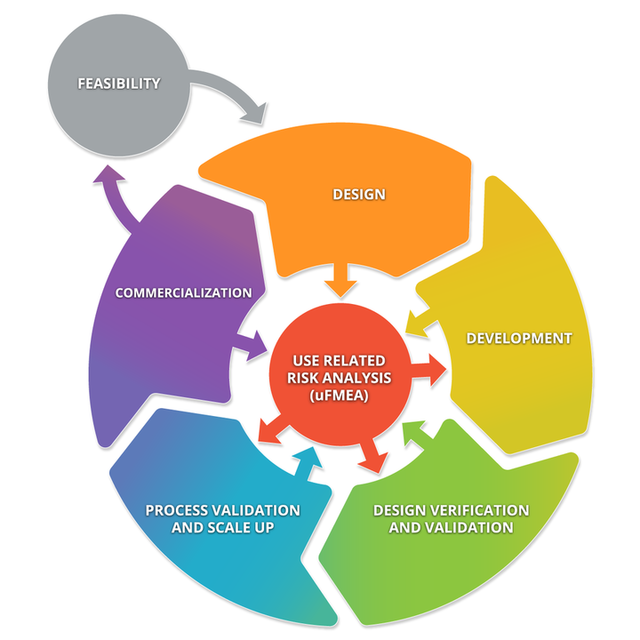
The diagram below depicts a typical product lifecycle. The subprocesses depicted in this diagram are: Feasibility, Design, Development, Design Verification and Validation, Processes Validations and Scale Up, and Commercialization. These subprocesses consistently interact with Use Related Risk Analysis (useFMEA). The interaction with use risk is the most important part of the product life cycle because it is the obligation of the manufacturer to place a medical device on the market that is safe and effective.
Medical Device Product Life Cycle and Human Factors Activities



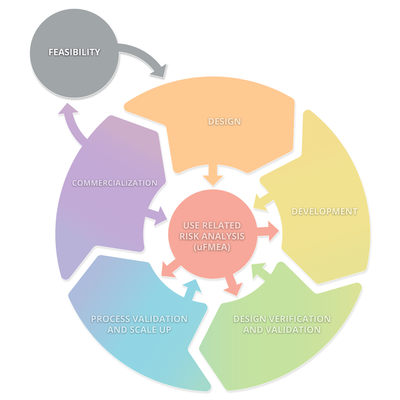
STAGE 0
Feasibility
Goal: The goal of the Feasibility sub process is to determine if the idea for the device is feasible from the perspective of various inputs. Namely, the following questions would need to be answered:
» Is there a user need to support the idea?
» What are the costs to get the device to market?
» What is the required time to get the device to market?
» Does the idea fit into an existing portfolio of products?
Human Factors Activities: Human Factors practices that are normally incorporated into this sub-process are concentrated on finding and defining the user needs for the idea. Typically, Human Factors researchers will conduct naturalistic observation/contextual inquiry sessions. In other words, there are visits to the prospective end users’ environment and observe their current work/tasks that they perform with existing related devices. In addition, in depth structured interviews could take place to understand the relative pain points that currently exist. This information is formulated into a User Needs document. Note, this sub process can be carried out in conjunction with related Marketing activities.
Stage 0: Feasibility
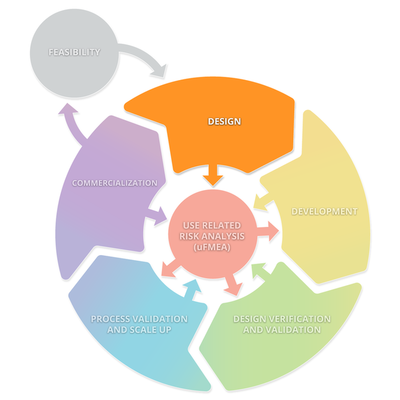
STAGE 1
Design
Goal: The goal of the Design sub process is to create an instantiation of the medical device that can be developed, i.e., a Design. There are several functions that contribute to this sub-process. They include but not limited to: Engineering, Human Factors, Regulatory Affairs, Quality, Marketing, etc
Human Factors Activities: The first Human Factors activity has to be an assessment of what will be needed to be accomplished in terms of Human Factors throughout the product life cycle. In other words:
» What documentation will be needed for the usability engineering file?
» What type of Human Factors testing will be required?
» What is the timing of the Human Factors testing?
This information is formulated into a Human Factors Activity Plan and/or Human Factors Evaluation Plan.
The next Human Factors activity is to create a Use Specification
document. This document will take information from Marketing, the User Needs document, and Engineering to answer the following:
» Who are the intended users, the intended environment, the
intended use?
» How does the proposed device respond to the User Needs?
» What are the system’s use requirements and use specifications?
» What are known use problems of existing devices or similar
devices?
» How does the proposed device “fix” those known use problems?
This Document is part of the Usability Engineering File.
The last Human Factors activity in the Design sub process is the creation of the Task Analysis. The information from the output of the Feasibility sub process, the User Needs, and the Use Specification are used to construct a Task Analysis. This is the proposed manner/steps that the user will utilize the proposed medical device. Note: The Task Analysis is basis for the useFMEA.
Stage 1: Design
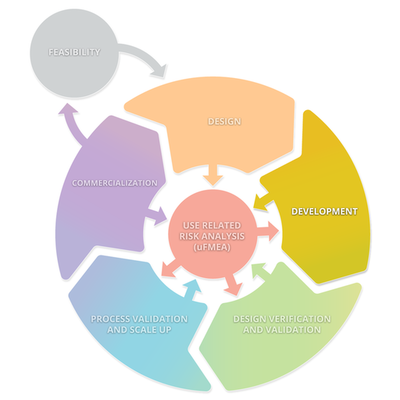
STAGE 2
Development
Goal: The goal of the Development sub-process is to create the medical device. This sub-process has several iterative steps that ensure the device being built will meet all requirements, e.g., users, marketing, resources, regulatory, engineering, quality, etc.
Human Factors Activities: There are two main activities for Human Factors researchers during this sub process.
1. Create the User Interface/Interaction Specification and/or a Product Prototype.
This document uses the information from the Use Specification, the Task Analysis, and the useFMEA (first version without design mitigations listed). In addition, there are several references that are referred to: e.g., AMERICAN NATIONAL STANDARD/AAMI HE75:2009/(R)2018 HUMAN FACTORS ENGINEERING – DESIGN OF MEDICAL DEVICES; W3C, IEC 62366, etc. One of the main purposes of the User Interface/Interaction Specification is to supply design mitigations for the use FMEA.
2. Implement Formative Human Factors testing
Human Factors formative tests typically take the information from the useFMEA and the User Interface/Interaction Specification and evaluates the parts of the device that have the potentially highest use risks. These are usually small, quick, and iterative. They are conducted prior to any development “freezes” as they are used to inform the final development.
Stage 2: Development
STAGE 3
Design Verification and Validation
Goal: The goal of the Design Verification and Validation sub-process is to perform a Human Factors Summative Test.
Human Factors Activities: The Human Factors Summative test is the final user testing that is completed with a production equivalent device and/or verified software. A report is created of the testing to include a complete testing protocol, all related materials, e.g., IFU, Training Protocol, etc. The results are used to update the useFMEA.
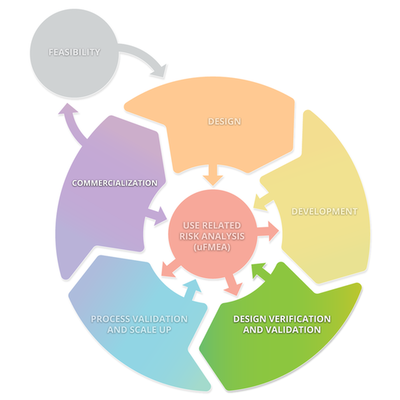
Stage 3: Design Verification and Validation
STAGE 4
Process Validation and Scale UP
Goal: The goal of the sub-process validation and scale up sub process is to create the Human Factors Summary Report. This report is submitted with the regulatory submission.
Human Factors Activities: The Human Factors Summary Report is a complete analysis of ALL Human Factors Activities conducted during the Product Life Cycle. This is the document that also indicates to the notified/regulatory body that the device is safe and effective when used by the intended users, for the intended purpose, in the intended environment.
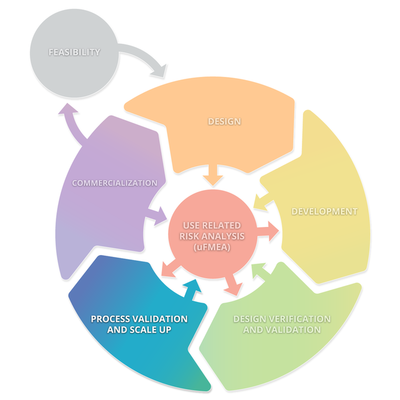
Stage 4: Process Validation and Scale UP
STAGE 5
Commercialization
Goal: The goal of the sub process commercialization is to bring the medical device to market and to diligently preform surveillance and vigilance to the success and issues of the medical device.
Human Factors Activities: The Post Market Vigilance Plan and Report is a proactive activity. The purpose is to periodically observe the intended users using the medical device in the intended environment for the intended use. The report will influence the useFMEA as well as being a catalyst for new devices.
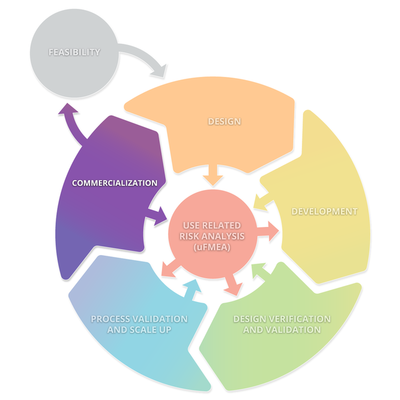
Stage 5: Commercialization

+1 (619) 301-2073
mdhf@hirlan.com
Medical Device Human Factors
c/o HirLan, Inc.
2121 Palomar Airport Road
Suite 202/203
Carlsbad, CA 92011
USA
