CaP Biomaterials
CaP Biomaterials manufactures calcium phosphate materials that can be incorporated into many types of medical devices by medical device manufacturers. Because of diversity of applications it is CaP Biomaterial’s policy to work with customers in the process of choosing the calcium phosphate product requirements/specifications. This often results in supplying a customized product exactly suited to the end use.
CaP and customer come to agreement on material requirements and preparedraft product specifications.
read more
watch the video
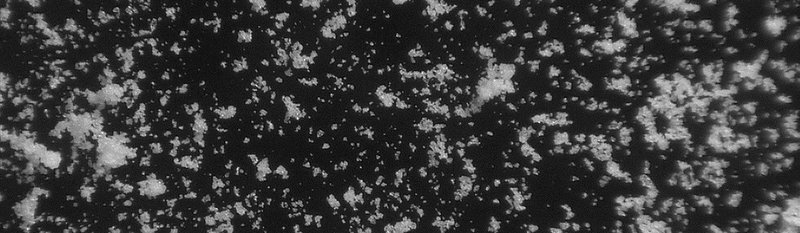
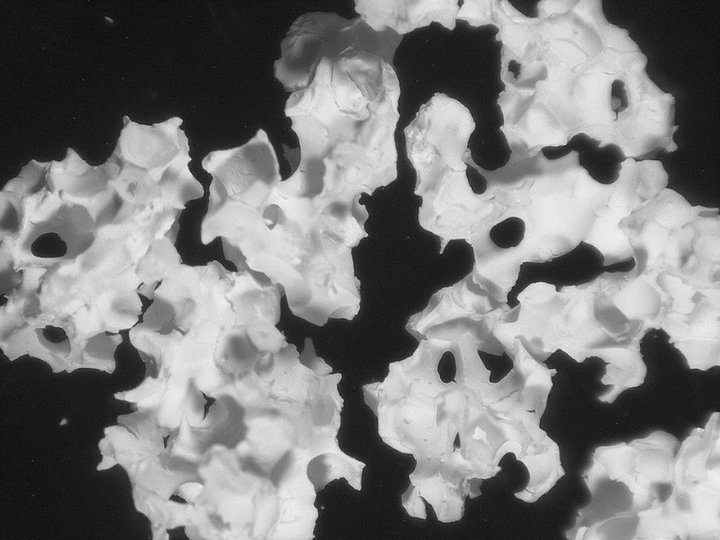
Hydroxyapatite porous granulate
Decide if an existing material is suitable or a custom material is needed.
- Porous granulates of:
- Hydrxyapatite
- Tricalcalcium phsphate
- Mixtures f hydroxyapatite and tricalcium phosphate
- Hydroxyapatite powder for plasma spraying
- Calcium phosphate powders of many different compositions for incorporation into polymers
- Metastable calcium phosphate powders (tetracalcium phosphate; alpha-TCP) for producing self-setting cements
- Finished self-setting cement powder
CaP Biomaterials prepares/supplies development samples so customer can test the concept.
CaP carries out extensive testing on the prototype product including:
- XRD analysis for phase composition
- optical image analysis for pore size, porosity, and particle size
- surface area analysis (if requested)
- dissolution and/or solubility (if requested)
- trace metals analysis
read more



Product specifications are discussed with customer and may be revised.

Prepare batches for customer to use in their process/product validation (if requested).
- Hydroxyapatite coated implant
- Collagen strip with ceramic particles
- Putty with ceramic particles
- Tray package filled with ceramic particles
read more
watch the video


