Company Insight
Sponsored by Alleima
Medical device startups continue to face complex material challenges
Innovation in medical devices continues to propel the market forward. From electrophysiology to remote monitoring and miniaturization, the evolving technical capabilities of the industry are enabling exciting new products to enhance patient treatment. At the same time, regulatory and supply chain pressures place scaling medical device companies under strain.
Main image: Forming partnerships with suppliers who possess in-depth materials and development expertise, as well as a nuanced understanding of the medical innovation landscape, is essential. Credit: Alleima

Dr. Bernd Vogel
Innovation in medical devices continues to propel the market forward. From electrophysiology to remote monitoring and miniaturization, the evolving technical capabilities of the industry are enabling exciting new products to enhance patient treatment. At the same time, regulatory and supply chain pressures place scaling medical device companies under strain.
Startups in the space must overcome a wealth of technical and logistical challenges to break into this crowded market. Regulatory hurdles, manufacturing errors, and supply chain disruption can all become overwhelming. Failure to comply with regulations and guidelines can prevent new products from reaching markets, as well as forcing startups to pay hefty fines they can’t afford to breach.
Nevertheless, innovation starts from the bottom. These small businesses require the right partners and support to ensure the medical device industry continues to move forward.

Braiding is one of Alleima’s core capabilities in nitinol processing. Others include laser-cutting, shaping, grinding, joining, and cleanroom assembling. Credit: Alleima
Nitinol's versatility has led to its use in a wide array of medical devices. In addition to guidewires, nitinol is used in flexible retrieval devices and instruments, vascular filters, and permanent implants. These devices benefit from nitinol's ability to endure repeated stress without losing shape or performance. Nitinol’s adaptability allows it to be crimped, bent, or coiled into tight configurations, easing insertion through minimally invasive methods. In cardiovascular treatments, nitinol filters capture blood clots and prevent embolisms. These filters collapse for deployment and expand inside blood vessels to perform life-saving functions. Nitinol is also promising in steerable medical devices like endoscopes and catheters.
However, manufacturing nitinol in a cost-effective way while maintaining the highest quality requires specialist capabilities. Even the slightest deviation during production can alter the performance of the material. Understanding the impact of cold work, heat treatment, strain rate, and number of cycles is essential for optimizing the stress-strain behavior of nitinol.
Having acquired long-standing nitinol expertise with Endosmart in 2022 and Endox in 2024, Alleima combines decades of expertise in material science with leading know-how in nitinol processing, delivering wire-based solutions and medical components designed to meet the exacting demands of modern surgery. Whether it’s developing steerable devices for robotic surgery or advanced, flexible instruments and implants for various applications, including urology, gastroenterology, oncology or cardiovascular, Alleima’s collaborative approach ensures its clients can navigate the complexities of nitinol manufacturing with ease, highest quality, and functionality of its solutions all the way to market approval.
In a field where every innovation can translate into saved lives and improved patient outcomes, access to expert knowledge is invaluable. To learn more about Alleima's engineering services and processing capabilities in nitinol, visit their website, Nitinol—shaping the future—Alleima, where you can also download their nitinol whitepaper Nitinol and its transformative role in medical devices.

Dr. Bernd Vogel

Braiding and Twisting detail center. Credit: Alleima
Complex outlook for medical startups
Regulatory hurdles often prove to be the greatest obstacle between medical startups and the market. These rigorous guidelines are frequently subject to change and vary drastically between different countries. In addition, lengthy approval processes frequently delay product launches, making it difficult for startups to generate revenue quickly.
Failure to comply with regulations can also result in startups getting saddled with avoidable fines, presenting further financial complications. Yet startups often lack the necessary personnel and expertise to meet all the required quality control standards. Regardless, they have to be certain their products meet the necessary safety and efficacy criteria.
Operational challenges present additional complexities. Medical device startups can lack knowledge about design or materials to use when they start a project, which later in the process has a significant impact on prototyping and finalising the product for production. Additionally, when scaling processes in line with demand, it can be difficult for suppliers to keep pace. Startups need to be working with partners who understand regulations inside-out, can offer necessary support on their growth journey, and can advice about potential pitfalls on the way to market.
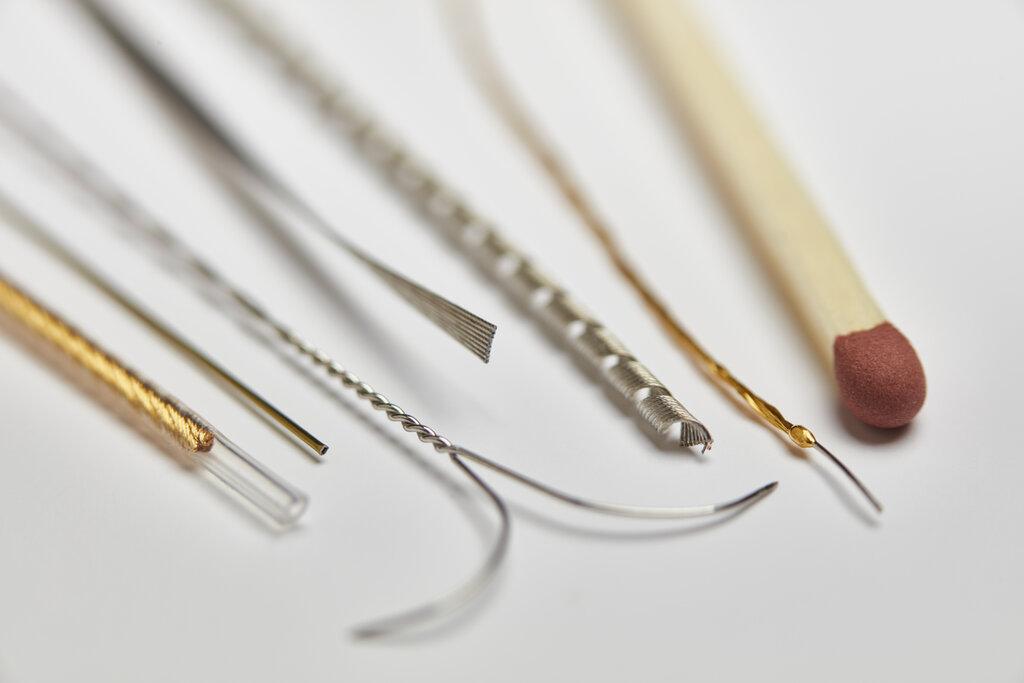
Alleima’s wire-based solutions have been incorporated into a diverse range of innovative medical devices from pacemakers and neurostimulators to hearing aids, ablation catheters, and guidewires. Credit: Alleima
When asked to simply describe the shape-memory effect, Dr. Bernd Vogel takes out a wire shaped like a heart, straightens it completely, and then slowly dips it into a cup of hot coffe. Immidately the wire returns to its perfect heart shape.
Enhancing the development lifecycle
Operational complexities pose unique hurdles for medical device startups. Early in the product development lifecycle, teams may require specialised knowledge about optimal materials or design features—decisions that can significantly impact successful prototyping and subsequent transition to scalable production. As a startup’s growth accelerates, it is not uncommon for production demands to outpace established supply capabilities, making it crucial to anticipate and manage process scalability from the outset.
Forming partnerships with suppliers who possess in-depth materials and development expertise, as well as a nuanced understanding of the medical innovation landscape, is essential. The right partners not only help navigate evolving market and compliance requirements but also provide technical guidance to avoid common pitfalls along the road to commercialsation. By collaborating strategically, startup R&D teams can foster flexible processes and build robust development pipelines that are equipped to adapt alongside business growth and technological advances.
Startups’ difficulties have been compounded by fluctuations across MedTech supply chains. Limited manufacturing capacity for cutting-edge devices is one challenge, while manufacturing bottlenecks continue to impede the flow of products to the market. Meanwhile, geopolitical tensions worldwide, including conflict and inflation, continue to pressurise supply chains that are already stressed.
Once again, a lack of expertise in these areas can seriously hinder a startup’s growth. The right partners are able to work with startups to overcome arising challenges and plan a strong strategy for supply that mitigates the many risks associated with global medical supply chains today.
An expert materials partner
For startups seeking exceptional, high-performance partners, Alleima brings leading material expertise, engineering know-how and adaptable vertical integration. The company’s extensive portfolio capabilities, materials and integrated solutions include ultra-fine wires, thermocouples, and complex nitinol components – all engineered to support the sensing, measurement, stimulation, transmission, capture, and cutting functions required by today’s most innovative medical devices. Alleima’s solutions have been incorporated into a diverse range of innovative medical devices, from pacemakers and neurostimulators to hearing aids, ablation catheters and guidewires.
An innovative acquisition strategy along with strong organic growth has meant that Alleima’s scope expands far beyond its headquarters in Sweden. With a global manufacturing footprint and dedicated engineering teams, the company is uniquely positioned to support growing international medical businesses. Alleima provides technical competence, precision manufacturing capabilities and production capacity to reduce lead times, accelerate design iterations, and improve operational outcomes, all while staying on top of the relevant regulations and ensuring they are supplying the best quality solution according to the customer’s needs.
With advanced in-house capabilities spanning fine-wire processing, nitinol processing, laser technologies, and specialised surface treatments, Alleima stands uniquely equipped to meet the evolving needs of medical device startups.
Meet Alleima at MD&M Midwest in Minneapolis
Meet our team at MD&M Midwest, Minneapolis, October 21-22, 2025, booth #3315.
Explore how Alleima can support your journey – from design and prototyping to commercialization.
Contact information
Alleima Advanced Materials
1 Commerce Blvd.,
Palm Coast, FL, 32164,
United States
Tel: +1 386 445-2000
Fax: +1 386 447-5113
Email: sales.pc@alleima.com
Alleima Sonceboz
Sur le Brassiège 3
2605 Sonceboz-Sombeval
Switzerland
Tel: +41 32 942 39 20
Email: sales.sb@alleima.com
Alleima Karlsruhe
Wilhelm-Schickard Str. 9c
761 31 Karlsruhe
Germany
Tel: +41 41 761 63 55
Email:sales.ka@alleima.com
Alleima Dettingen
Paul-Lechler-Str.14
72581 Dettingen/Erms
Germany
Tel: +49 7123 91019-0
Email: info.det@alleima.com