COMPREHENSIVE SERVICES
UNCOMPROMISING QUALITY
The PSN Test Lab is equipped with industry leading technology for risk assessment and qualification of devices, as well as the generation of data for FDA and other notified body submissions. Our team of scientists and engineers are trained to isolate confounding variables and provide clear analysis to guide the commercialization of new medical devices.
Integrated Approach
Our state-of-the-art Testing Laboratory is ISO/IEC 17025:2017 accredited and fully equipped to support all your material and product testing needs. Our lab operates as a stand-alone service, as well as being integrated into our full spectrum of engineering services, which covers the full arc of the product development lifecycle. PSN’s experience and expertise crosses over a wide range of industries, products, and manufacturing processes, allowing our team to be a best-in-class choice to help determine and correct the risks associated with device manufacturing.

Chemical Characterization
ISO 10993-18:2020
Key inputs into the Toxicological Risk Assessment of a medical device include Chemical Characterization after Extractables and Leachables Testing. The incorporation of additives to suit device applications often includes plasticizers, stabilizers, colorants and processing aids, all of which can impact patient safety. Extractables and Leachables testing enables the determination and quantification of chemical compounds that may leach and /or extract out of a medical device during use. This in turn can inform the overall biological risk assessment of the device and its impact on patient safety, including if additional biological testing is warranted.
PSN has developed an advanced process for Extractables and Leachables Testing, which is offered under our ISO/IEC 17025:2017 accreditation scope. Our scientists and SMEs actively monitor FDA feedback through published literature to incorporate a revised approach.
PSN conducts the full suite of analytical testing which includes:
- Volatiles and semi-volatiles via Gas Chromatography-Mass Spectrometry
- Non-volatiles via Liquid Chromatography-Mass Spectrometry
- Elemental impurities via Inductively Coupled Plasma Mass Spectrometry
PSN’s toxicology team works in harmony with the analytical team to deliver the final report with a toxicological risk assessment.
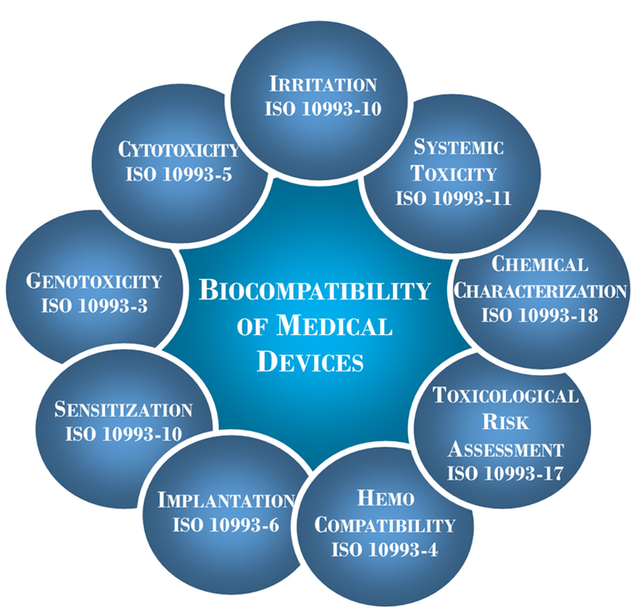
Biocompatibility Testing
Utilizing the concepts and principles outlined in ISO 10993-1:2018, a comprehensive and custom biocompatibility test plan is created dependent upon your unique medical device classification.
Once all biological endpoint testing has been conducted, a comprehensive biological risk assessment is created that references all ISO 10993 testing, along with ISO 18562 (if applicable) in order to support 510 (k) submissions, notified body submissions, post-market product changes, and/or new product development.
Toxicological Risk Assessment
ISO 10993-17:2002, ISO/TS 21726:2019
The toxicological risk assessment is a critical component of a comprehensive biological risk assessment. This analysis utilizes concepts to include health-based safety thresholds, tolerable intakes, tolerable exposures, and threshold of toxicological concern (TTC) in order to derive a risk estimation and hazard characterization of either leachates, extractables, or volatile emissions from a medical device.
All toxicological risk assessments are customized to the type of device, patient use case, and patient age/weight in order to give an accurate, yet conservative estimate of toxicological risk that may prompt further biological evaluation of the medical device, or be used in conjunction with other biological endpoints for a comprehensive biological risk assessment.
ISO 10993 Testing
Biocompatibility Evaluation
of Medical Devices
PSN is an ISO 9001:2015 certified engineering firm, providing services in all areas of product development. We have three main Laboratories comprised of our Engineering Design Center, Material Processing Lab, and our ISO/IEC 17025:2017 certified Testing Laboratory. Our teams of highly qualified engineers, scientists, and SMEs work across each lab to provide a unique advantage to our clients.

Email: info@PSNLabs.com Phone: +44 814.464.0790 Fax: 814.464.0794
