Project Updates
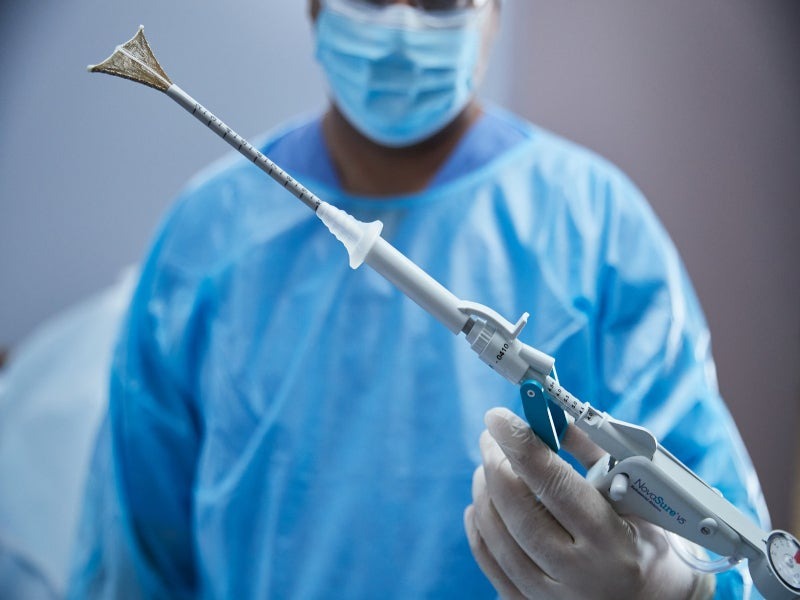
Hologic’s NovaSure V5 Global Endometrial Ablation System, Europe
Credit: Hologic
Project Type: Global endometrial ablation (GEA) device
Developer: Hologic
Applications: Laparoscopic treatment for abnormal uterine bleeding (AUB) in women
Status: Approved in Canada and Europe
NovaSure V5 is an innovative global endometrial ablation (GEA) device developed by the US-based medical technology company Hologic. NovaSure V5 was launched in the US in November 2021.
CoreLink’s Siber Ti Sacroiliac Joint Fusion System, US

Credit: CoreLink, LLC.
Project type: Sacroiliac joint fusion system
Developer: CoreLink
Applications: For sacroiliac joint fusion by surgical procedures
Status: Approved in the US
The Siber Ti Sacroiliac (SI) Joint Fusion System is a family of fully porous, nano-surfaced, 3D-printed implants developed by CoreLink, to support surgeons in SI joint fusion procedures. It received 510(k) clearance from the US FDA in March 2023.
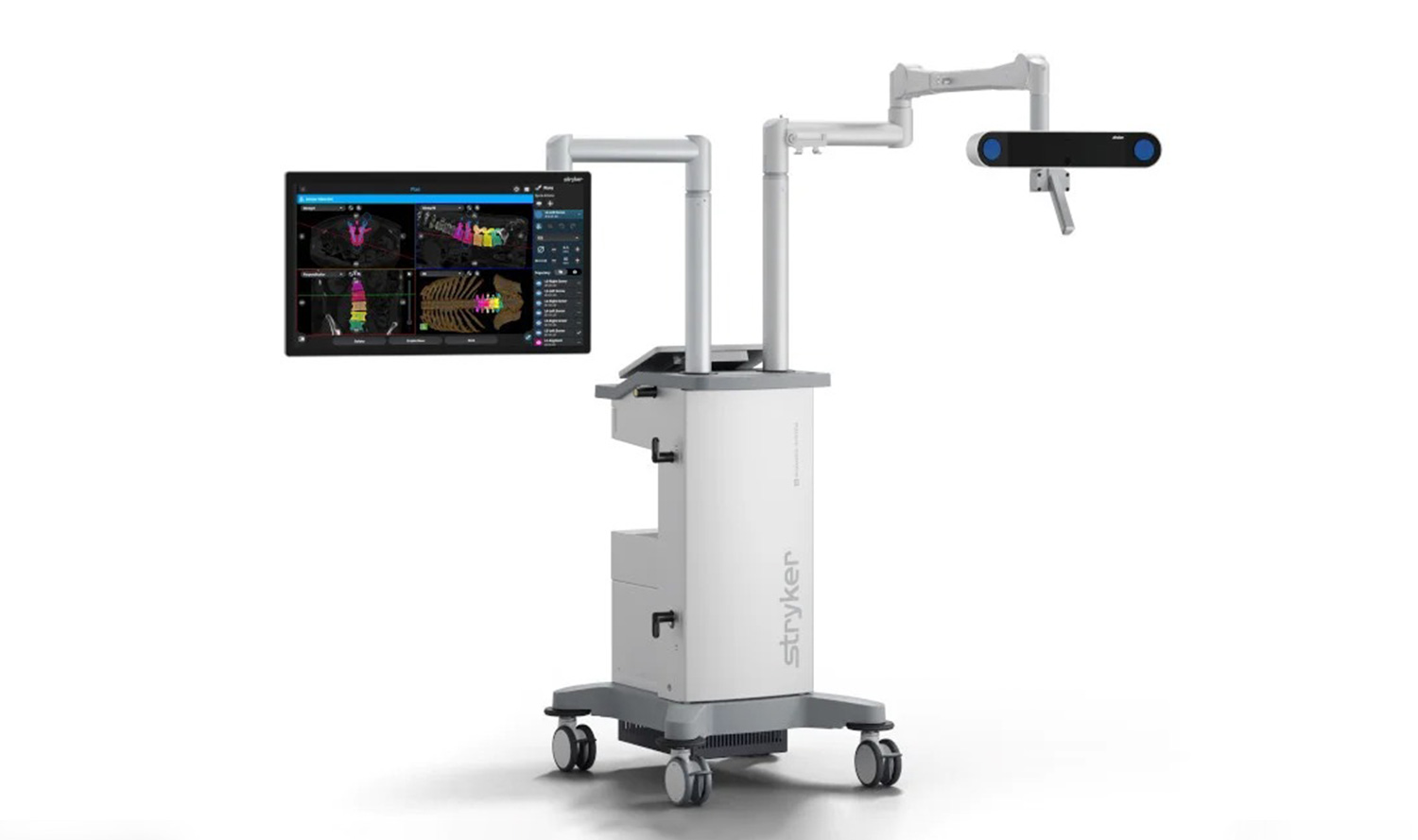
Stryker’s Q Guidance System, US
Credit: Stryker
Project type: Image-based planning and intraoperative guidance system
Developer: Stryker
Applications: To support cranial and spinal navigation and surgeries
Status: Approved in the US
The Q Guidance System is an image-based planning and intra-operative guidance system developed by Stryker, to support spine and cranial surgery procedures. It received 510(k) clearance from the US FDA in June 2022.