Image: Ali Tamayol
Q&A: Innovation
Are ‘smart’ bandages the future of wound care?
Effective treatment options for chronic wounds are limited, but the consequences of poor management can be devastating and even lead to amputation. Natalie Healey speaks to Dr Ali Tamayol, from the University of Connecticut, who has developed a wirelessly controlled bandage that precisely delivers a combination therapy deep into the wound to encourage healing.
Chronic wounds are a distressing problem for millions of people. They particularly affect diabetic patients and are a leading cause of limb amputation. But wound healing is a complex process and clinical treatments are limited.
Dr Ali Tamayol from the Department of Biomedical Engineering at the University of Connecticut has developed a device that could help. Equipped with miniature needles that can be controlled wirelessly, the device allows medication to penetrate deep inside the wound bed. The treatment can be administered from a smart phone-like device without a healthcare provider having to even visit the patient. It has so far been tested in cell lines and mice, but it’s hoped the technology will replace current wound management practice in humans in the future.
Natalie Healey
What are your research interests?
Dr Ali Tamayol
I did my education in mechanical engineering, but during my postdoc I switched gears and moved towards biomedical research. Currently, my research has two major focuses. One is finding strategies to bring new technology to wound care practice. The other is developing tools to treat musculoskeletal injuries.
Why are chronic wounds so difficult to treat?
Dr Ali Tamayol
One of the challenges is that there is a need for continuous visits so wounds can be continuously monitored. It is inconvenient for the patients and right now in a situation such as Covid-19, as going to medical facilities frequently could be risky. Part of our focus was trying to minimise that as much as possible.
Another aspect is that doctors visually inspect the wound and do swabbing to look into whether there's contamination, but they don't have much information available to them to see what the status of wound is and how it’s changing underneath the surface.
Over the years, a lot of treatments have been successful in animals. But when it gets to humans, they are not as effective as you would think. Despite all the advances that have been made, diabetic ulcers are still the leading cause of amputation.
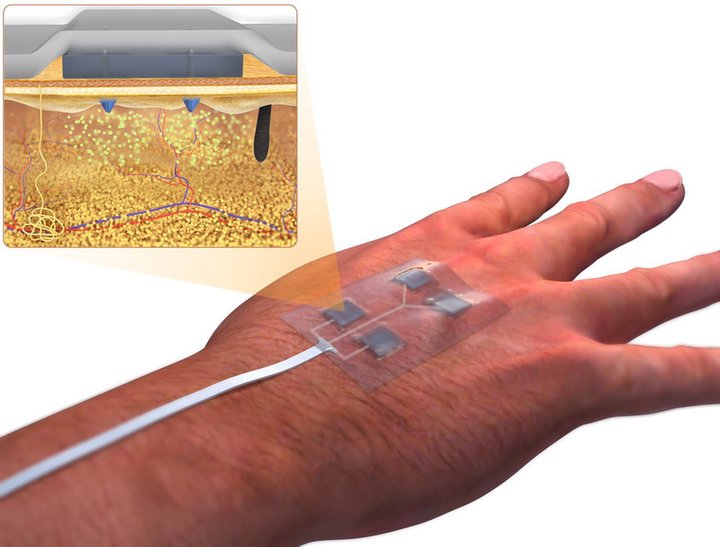
The bandage is equipped with miniature needles that can be controlled wirelessly. Image: Ali Tamayol
How does your system work?
Dr Ali Tamayol
We have developed different versions over the years. The first version was about being able to actively release medications. The second version was pairing that with sensory systems. But then we realised that in the majority of wound management cases, we don't need one single drug, it must be multiple drugs. So, then we tried to change the system so it could deliver different drugs with independent release kinetics where the care provider has a full control over the system.
Typically, wounds are covered by a layer of necrotic tissue. So, if you apply medication on top, the bioavailability of the medication around the cells that need to respond to it is going to be low. We try to cut down that distance by using miniaturised needles which can penetrate the wound bed and then release the medication.
We combine these two concepts in a system which is widely controlled and programmable and precisely delivers the right drug at the desired time, at the desired quantity.
How effective has the system been?
Dr Ali Tamayol
We initially tested it on human cell lines. And then once we were sure that the materials were safe, we did a pilot animal study on diabetic mice. In the mouse, the results were very interesting because we used a molecule called VEGF (vascular endothelial growth factor) as the treatment. It is one of the first molecules that the body produces to induce vascularisation, which is one of the processes that is essential for supporting wound healing. And it is widely accepted that in diabetes patients, vascularisation is slower than in a healthy population.
We treated two groups of mice. One we delivered topically and the other with the microneedle device, as well as having animals that were left untreated as our negative controls. What we noticed was that when we delivered medication through our system, it led to a faster recovery in the animals.
What’s next for the device?
Dr Ali Tamayol
Going straight to human tests is not the best idea because rodent skin healing is fundamentally different from human skin. But there are other animals that have a comparable healing process to us. We are trying to start an experiment with pigs or other large animals and see if we can verify the results we found in mice. And if the results are promising and everything makes sense, then we should think about the next steps.
There is a significant need for screening different types of wound and understanding which ones this device could particularly be effective for. But potentially it could one day replace regular bandages.
What are your hopes for the future of wound care?
Dr Ali Tamayol
Whenever I publish a paper, I get lots of emails from chronic wound patients. Many want to volunteer to test out the device. Seeing these emails, seeing people suffering and talking to care providers makes you just want to develop something that will help.
It doesn’t matter if it’s our group or another research group who gets there. As long as we can develop technology that will ensure more limbs are going to be saved and people have to go through less pain and they’re going to have a better life, I’ll be happy. When we go to the lab, that is our motivation.