SOLUTIONS FOR
YOUR PROJECTS
ISO 13485:2016 CERTIFIED
MEDICAL DEVICE CONSULTING SERVICE
Who We Are
We Are a Team of Engineers and Subject Matter Experts with a long experience in the Medical Device field, this allow us to quickly find the right approach for the project, optimizing development basing on regulatory field, applicable international standards and previous experience with the biggest market leader companies.
Customized Solution:
We support our clients on a specific service or on a whole project development. We tailor our solution on client needs.
MDR & Regulatory Support





MDR 2017/745 CONSULTING SERVICE
With our team of SMEs and Regulatory experts we provide support to companies in different fields and with different kind of products going through the changes introduced by new MDR.
We will start with a Gap Analysis to assess your current level of compliance and highlight gaps.
Then we will define a proper MDR Transition plan to help you navigate trought the change and reach safe the harbour of MDR compliance.
We can help you out and support on hot MDR topics such as:
- CLASSIFICATION AND CONFORMITY ROUTE
- NOTIFIED BODY SELECTION
- UDI / EUDAMED
- TECHNICAL FILE
- CLINICAL EVALUATION
- PMS PROCESS
- QMS GAP ANALYSIS
- SOFTWARE AS MEDICAL DEVICE
- 3D PRINTED MEDICAL DEVICE
- COMBINATION PRODUCTS
We provide also:
- EU Authorized Representative
- UK Responsible Person
- Swiss Authorized Representative
- PRRC Experts;
TS QUALITY & ENGINEERING
We work for our client in order to get their product on the market efficiently and effectivly. We provide a full range of regulatory support service, with particular focus to:
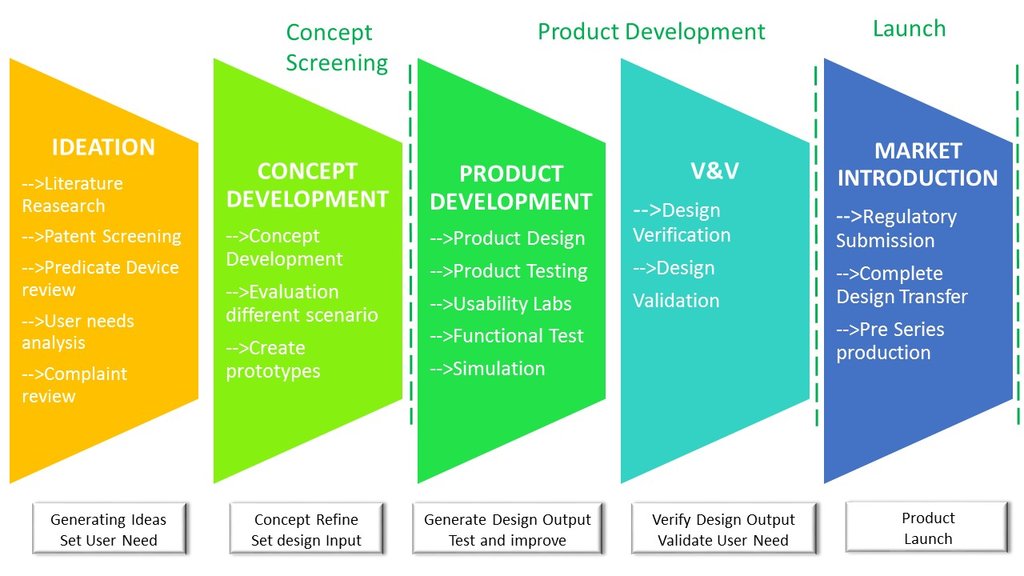
From ideation to final market introduction.
We are ISO 13485 certified for Medical Device Design, Development and all related activities.

From Concept Design to the Final device
Medical Device Design and Development
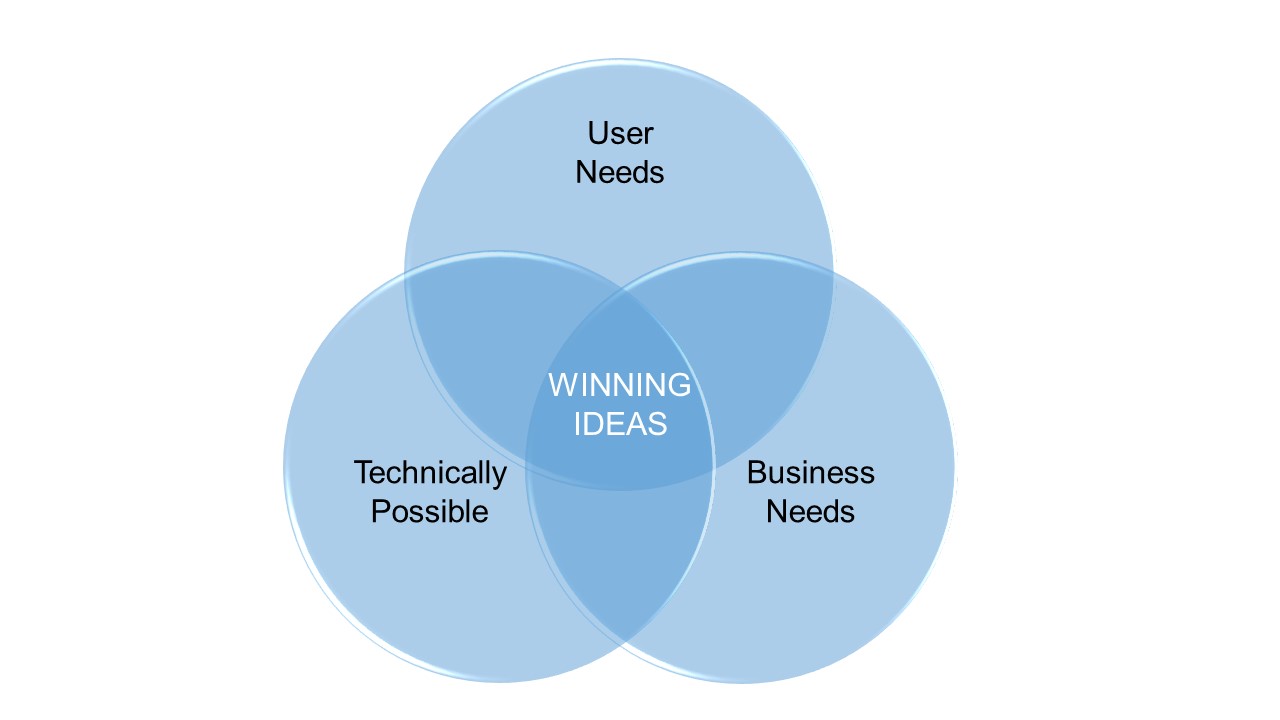
We can support and guide trought all the develoment process. Defining and addressing the User Needs, matching Technical possibilities to Business needs to find best solutions and finally develop a successful product!
We provide support to our clients in getting trought all the product development stages.
From ideation to final market introduction.
We are ISO 13485 certified for Medical Device Design, Development and all related activities.
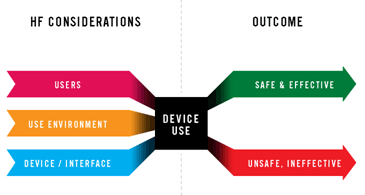
Usability & Human Factor Engineering Consulting
Our Service
Full regulatory compliance to EU and USA requirements- ISO 62366, FDA Guideline
- Risk assessment of human factors
- Wide variety of usability assessment tools
From standard review to user testing, from heuristic analysis to time-and-motion studied
- Design and re-design (device & information for use)
- Usability validation (summative & formative)
Field of Application of HFE expertise and support:
- Medical Devices in All Healthcare Domains
- Medical Device Accessories
- Websites and Mobile Applications
- Health Information Technology
- Documentation and Training
- Anthropometrics and Accessibility
- Signage and Labeling
- Instructions for Use
Reach out to us now and we will be able to support you!
Quality & Validation
QMS and Process Validation Support
We support establishment of QMS Quality Management Systems for the Medical Device and Pharma sector in compliance to ISO 13485:2016 and cGMP standard.
We provide our experience and support in order to:
- Setting up ISO 13485:2016 QMS
- Prepare for MDSAP Audit
- Perform Supplier and Internal Audit
It is necessary to show that there is full control over all life cycle of a product starting from the ideation, the definition of constraints and specifications, the choice of the design, the production process.
Process Validation is key for reliable manufacturing
We can support in
- Performing IQ/OQ/PQ process validation
- Drafting GAMP5 documentation eg. URS, FDS, HDS, SDS
TS QUALITY & ENGINEERING
SMART TOOLS FOR COMPLIANCE
We provide top-level consultancy and also the digital tools to streamline compliance and save time and costs
UDI EUDAMED TOOL
Web App
- Dedicated back-end
- Revision management
- Fill all UDI-DI field
- Fill all Basic-UDI field
- Dashboard for easy monitor UDI status
- Easy search
- Duplicate function
- Export XML for EUDAMED (optional)
- Blockchain Audit Trail (optional)
E-IFU

Web App
- Dedicated back-end
- Revision management
- QR scan and download
- Language management
- Local language recognition
- Blockchain Audit Trail (optional)
E-QMS

Modules available:
- Design Control
- Document Control
- Equipment Management
- Electronic Signatures
- Product Management
- Post-Market Surveillance
- Risk Management
- Supplier Management
Validate means
“Providing an evidence documented by ensuring, with a high degree of safety, that a specific process (or subprocess) is able to make in a repeatable way a product that meets the specifications recorded and the quality standards predetermined”
We perform validation of following processes
- Injection Molding
- Pharmaceutical Packaging
- 3D Printing
- CNC Milling
- Clean rooms
- Sterilization processes
- Cleaning Processes
- Heating treatments
- Coating Treatments
It ‘necessary to show that there is full control over all life cycle of a product starting from the ideation, the definition of constraints and specifications, the choice of the design, the production process.
It ‘important to confirm that in all these phases a repeatable method has been used and demonstrate through records, documentation, procedures.
The choice of the validation procedure, the tests for the validation and related acceptance criteria must be defined based on reference standards and optimized according to the characteristics of the product or process that you want to validate.
We support the validation and the establishment of quality systems with particular reference to the medical device sector, with reference to ISO and GMP standards.
WE WILL HELP YOU CREATE A SUCCESSFUL PRODUCT
We have huge experience in medical device design & development with particular focus to following fields:
Orthopaedics
Traumatology
Neurosurgery
Urinoginecology
Cardiovascular
Surgical Instruments
It ‘necessary to show that there is full control over all life cycle of a product starting from the ideation, the definition of constraints and specifications, the choice of the design, the production process.
It ‘important to confirm that in all these phases a repeatable method has been used and demonstrate through records, documentation, procedures.
The choice of the validation procedure, the tests for the validation and related acceptance criteria must be defined based on reference standards and optimized according to the characteristics of the product or process that you want to validate.
We support the validation and the establishment of quality systems with particular reference to the medical device sector, with reference to ISO and GMP standards.
6 STEPS FOR THE EU MDR GAP ANALYSIS
1.CLASSIFICATION (CHAPTER V)
- Check new classification rules (MDR Classes I, IIa, IIb and III) (Art. 51, Annex VIII) (eg. Surg mesh, software,etc…)
- Check the new definition of MDR, particularly with respect to its expanded scope. This also applies to products covered in (Art. 1, 2, Annex XVI) (eg. Cleaning device, Contact lens, liposuction, skin treatment…)
- Special Device (Art. 21) (investigation dev., etc..)
2.CONFORMITY ASSESSMENT (CHAPTER V)
- Confirm conformity assessment routes for existing and future products (Art. 52, Annex IX, X, XI) (eg. Class Ir..)
3.CHECK NOTIFIED BODY STATUS
4.REVIEW QMS AND TECHFILE
QMS
- Review and upgrade quality management system (QMS) (eg. Art. 10 (9))
- Assess impact on products, internal resources, organisation and budget, ex. Person responsible for regulatory compliance (Art. 15)
TECH FILE
- Review the changes needed to existing technical documentation (Annex II)
- Tech file conservation period (min. 10 yr after last device placed on the market Art.10 (8))
5.PERFORM PMS (Chapter VII) and CLINICAL EVALUATION AND INVESTIGATION (Chapter VI) GAP ASSESSMENT
6.EVALUATE NEW UDI AND TRACEABILITY REQUIREMENTS
EUDAMED/UDI AND TRACEABILITY
- Ensure the respect of traceability obligations UDI/EUDAMED (Chapter III)
- Information to be provided upon registration (Annex VI)
LABELLING
- Review product labelling (Annex I ,Chapter III))
- Implant card and information to be supplied to the patient with an implanted device (Art. 18)