IN FOCUS Excellence Awards Interview
Sponsored by THINK Surgical
Medical Device Network Excellence Awards 2024: THINK Surgical
THINK Surgical is based in the U.S. and is a Dual Category Award Winner in the 2024 Medical Device Network Excellence Awards
THINK Surgical, a U.S.-based developer of orthopedic robots, has won the 2024 Medical Device Excellence Awards in the Innovation and Product Launches categories for its innovative approach to orthopedic surgery.
The Medical Device Network Excellence Awards celebrate the greatest achievements and innovations in the medical devices industry. Powered by GlobalData’s business intelligence, the Awards recognize the people and companies that are driving change in the industry.
THINK Surgical won the Innovation award for its TMINI® Miniature Robotic System, a first-of-its-kind miniature, handheld, wireless robot that enhances the precision and efficiency of orthopedic surgery. The Product Launches award was secured for the strategic and successful introduction of the TMINI System, which demonstrated adaptability and market demand by rapidly expanding its Implant Data Hub (ID-HUB™) and securing key partnerships within a tight regulatory timeline.
Medical Device Network: Can you describe the core innovation behind the TMINI® Miniature Robotic System that led to its recognition in the Innovation category?
THINK Surgical has successfully miniaturized a robot for total knee replacements. The TMINI System is a handheld, wireless robot which supports total knee arthroplasty
procedures by placing pins in planned planes on the femur and tibia to enable accurate bone resections. It allows the surgeon to complete the TKA procedure with the instruments they know and trust and removes the need for the cumbersome robotic cutting arm in traditional systems. This is only possible through incredible innovation and technological development. The TMINI Robot has completely reimagined the concept of robotic knee surgery.

Stuart Simpson, CEO
The TMINI Robotic System includes several major innovative breakthroughs in the handheld device itself and in the navigation console and camera technology which locate the robot and the patient anatomy to precisely deliver the surgical plan approved by the surgeon. Surgeons in our limited market release on average found the TMINI System ‘better’ or ‘much better’ to achieve their planned result than their standard of care, and unlike existing robots, the TMINI System is not tied to a single brand of implant.
THINK Surgical believes that the surgeon should be able to choose the most appropriate implant for each patient which is why we have partnered with 11 different implant
manufacturers to maximize surgeon choice. This is in stark contrast to the current robots which only offer the robot manufacturers brand of implants.
Medical Device Network: How does the proprietary 6-camera overhead active tracking system in the TMINI System enhance surgical efficiency compared to traditional 'line of sight' systems?
Surgical efficiency was a critical demand for the design of the TMINI System. The 6-camera overhead active tracking system plays a crucial role in meeting this requirement. By moving the camera overhead and adding 6 cameras for added redundancy to help avoid line of sight interruptions, we’ve enabled unrestricted patient access and minimized the disruption to OR staff caused by traditional camera systems.
Robotics and navigation users can relate to the frustration of interrupted tracking and the delays this causes during surgery. The TMINI System’s 6-camera tracking system builds in a level of redundancy which allows continuous tracking even if one or more cameras are blocked.
In addition, unlike traditional robots which use passive tracking technology, the TMINI System includes active tracking technology which improves overall system responsiveness. It is also resistant to blood and surgical debris that can be disruptive to a passive tracking system.
Medical Device Network: Accuracy is critical in orthopedic surgery. How does the TMINI System ensure sub- millimeter and sub-degree accuracy and precision?
THINK Surgical believes preoperative imaging is necessary to ensure optimal surgical accuracy and precision. The TMINI System uses the most advanced preoperative imaging available – computed tomography (CT) imaging – as the basis for planning and executing a precise surgery.
The sub-millimeter and sub-degree accuracy and precision of placing a cut guide compared to the planned placement showcases that a miniature, handheld robot, like the TMINI System, can reduce the size of technology in the OR without compromising on accuracy and precision.
Medical Device Network: The TMINI System is noted for its open implant platform. How important was this feature in your strategic planning, and what benefits does it offer to surgeons andpatients?
Bringing the TMINI Miniature Robotic System to market as an open implant platform was an incredibly important element in our strategic thinking. THINK Surgical is a robotics company, not an implant company. We work with our implant partners to bring the benefits of the choice of implant designs to patients, surgeons and administrators.
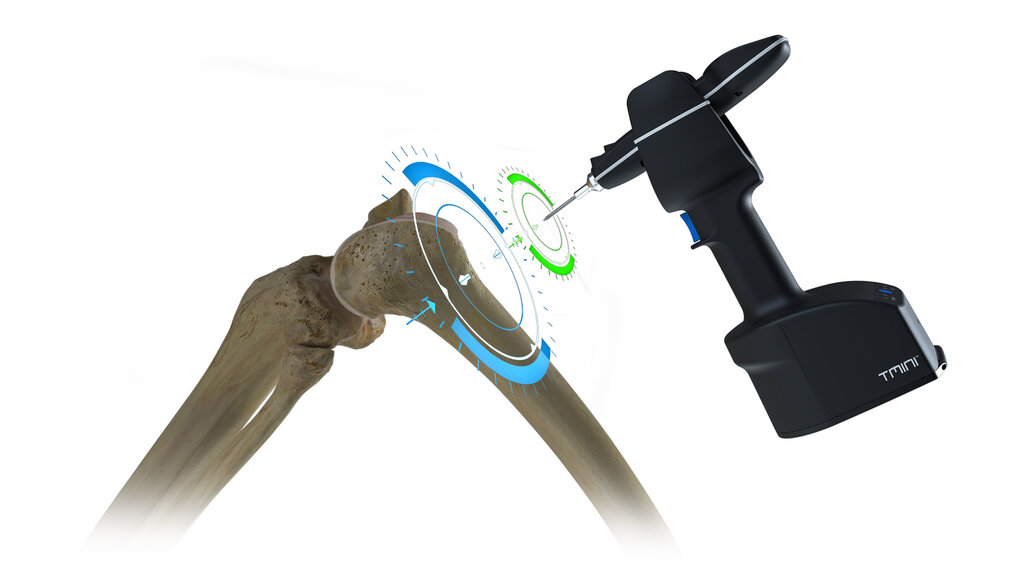
Colored graphics are for demonstration only.
This model allows facilities which use a variety of different implant systems, as most hospitals do, to only have to adopt a single technology to achieve a successful robotic total knee arthroplasty program. Surgeons are now able to select an implant from THINK
Surgical’s ID-HUB databank to optimize treatment based on their patient’s unique needs, rather than being stuck with a single implant option as they are with existing robots. At THINK Surgical, we believe in empowering surgeon choice.
Medical Device Network: Could you elaborate on the challenges and successes experienced during the tight timeline of regulatory approvals for the TMINI System?
Bringing a robotic total joint system to market is no easy task. The initial 510(k) clearance in May of 2023 was a culmination of almost 8 years of hard work. Since that initial clearance, our team has successfully achieved 510(k) clearance to bring our new TMINI System Version 1.1 to market and successfully achieved 510(k) clearance for use of the TMINI System with multiple additional implant systems. Each new TMINI System Version release and each use of the TMINI System with an additional implant system is subject to review and clearance by the FDA.
The THINK Surgical team successfully overcame many challenges during these highly coordinated releases. Meeting an aggressive timeline is always a challenge, especially when you need to coordinate efforts with external partners.
Medical Device Network: The collaboration with Zimmer Biomet and other implant manufacturers seemspivotal. How do these partnerships impact the market reach and adoption of the TMINI System?
Our current implant partners account for approximately 40% of the market and their sales teams are actively promoting the TMINI Miniature Robotic System as an enabling technology for use with their implants. This gives us access to well over 1,000 sales reps, which allows us to compete with the market-leading companies for scale. We believe that this network of partner salespeople gives THINK Surgical the opportunity to access 100% of hospitals and ASCs performing TKAs in the US. Since we don’t sell implants, these partnerships are mutually beneficial.
Medical Device Network: With the release of the TMINI System Version 1.1 and associated software update, what significant enhancements were introduced, and how do they reflect the company's commitment to continuous improvement?
In the past year, we’ve completed a limited market release of the TMINI System Version 1.0 with a select group of surgeons to help ensure the technology is ready to scale.
The TMINI System Version 1.1 release includes a significant software update that introduces the ability to perform a dynamic assessment of the patient’s compartmental gaps in the operating room and then make fine adjustments to the surgical plan before making any bone cuts. This functionality assists the surgeon to achieve a well-balanced knee for the patient without having to make any soft-tissue releases.
Completing the design, testing and clearance for the TMINI System Version 1.1 in under a year is a significant achievement. The TMINI Miniature Robotic System with the TMINI PRO Workflow (TMINI 1.1) is competitive with any TKA surgical robot on the market.
Medical Device Network: In what ways has THINK Surgical engaged with the medical community for clinical validation and peer endorsement of the TMINI System?
THINK Surgical continuously collaborates with the medical community for clinical validation through all stages of the product life cycle. We worked with our Medical Director, Scientific Advisory Board and early adopting surgeons through the development, testing and early clinical use of the TMINI Robot. We continue to work with them to optimize product performance and user experience.
Medical Device Network: Looking ahead, what are THINK Surgical's goals for the future of robotic orthopedic surgery, and how do you envision the TMINI Miniature Robotic System evolving tomeet those goals?
THINK Surgical’s goal is to make robotic knee surgery accessible to more surgeons and more patients. We are doing this by miniaturizing the technology and making it easier to use. By simplifying the technology without compromising on critical capabilities, we are making it easier for surgeons to adopt robotics.
We are also committed to an open platform business model allowing surgeons to choose from a broad range of implants to address individual patient needs.
Contact information
THINK Surgical, Inc.
47201 Lakeview Blvd.
Fremont, CA 94538
Tel: +1.510.249.2300
Email: marketing@thinksurgical.com
Web: www.thinksurgical.com
MKG-24-81 Rev01, MKG-24-88 Rev01
