Project Updates
TrueBeam Radiotherapy System, US

Project type: Linear accelerator radiotherapy system
Developer: Varian Medical Systems
Applications: Stereotactic radiosurgery and precision radiation therapy for tumours
Energy used: 2.5MV, 4MV to 25MV
Development Status: Approved in the US, Europe, China and Japan
TrueBeam is an advanced radiotherapy system developed by Varian Medical Systems, a medical device company based in the US, for delivering powerful and more accurate cancer treatments.
Monarch eTNS System, US

Project type: eTNS system
Developer: NeuroSigma
Applications: Treatment of ADHD
Technology: Trigeminal nerve stimulation (eTNS) system
Development Status: Approved in the US, Canada, Europe, and Australia
NeuroSigma’s Monarch external trigeminal nerve stimulation (eTNS) system is a non-pharmaceutical monotherapy for the treatment of attention deficit hyperactivity disorder (ADHD) in paediatric patients aged seven years to 12 years.
BD Alaris Infusion System, US
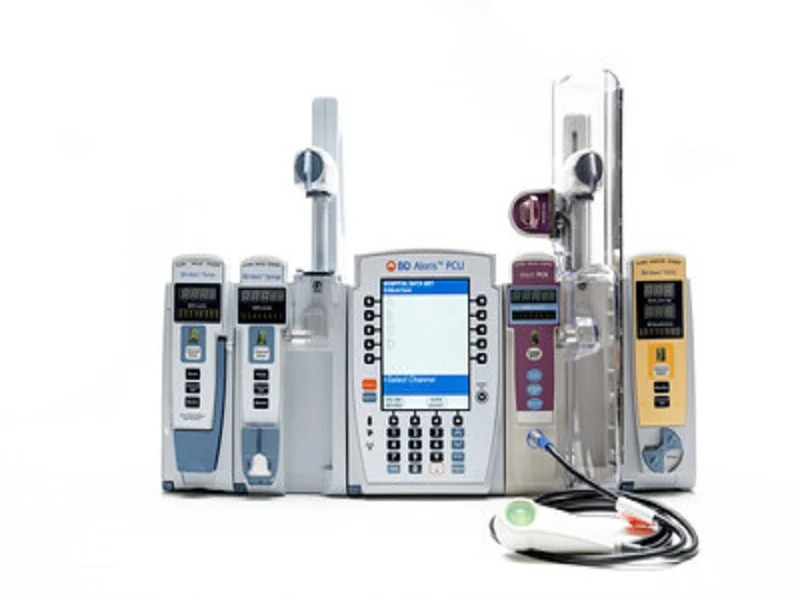
Project type: Infusion system
Developer: Becton, Dickinson and Company (BD)
Technology: Infusion system
Applications: Supporting smart infusion therapies across diverse care settings such as oncology, critical care, surgery, NICU and PICU
Development Status: Approved in the US
The BD Alaris Infusion System is a comprehensive, modular infusion system used for the delivery of fluids and medications.